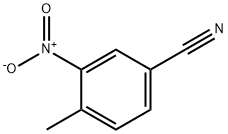
4-Methyl-3-nitrobenzonitrile synthesis
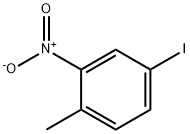
- Product Name:4-Methyl-3-nitrobenzonitrile
- CAS Number:939-79-7
- Molecular formula:C8H6N2O2
- Molecular Weight:162.15
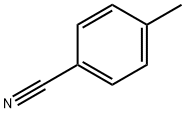
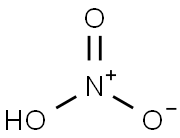
104-85-8

939-79-7
A. Synthesis of 4-methyl-3-nitrobenzonitrile. Nitric acid (20 mL) was slowly added dropwise to a mixture of 4-methylbenzonitrile (11 g, 0.098 mol) with concentrated sulfuric acid (20 mL) over a period of 1 hour at 0°C. After the dropwise addition was completed, the reaction mixture was continued to be stirred at 0°C for 1 hour. Subsequently, the reaction mixture was carefully poured into crushed ice to quench the reaction. The precipitate precipitated was collected by filtration to afford 4-methyl-3-nitrobenzonitrile (15.2 g, 95% yield) as a white solid. The product was characterized by 1H NMR (500 MHz, CDCl3): δ 8.27 (d, J = 1.6 Hz, 1H), 7.78 (dd, J = 8.0,1.7 Hz, 1H), 7.51 (d, J = 8.0 Hz, 1H), 2.69 (s, 3H).

104-85-8
328 suppliers
$11.00/25g

939-79-7
202 suppliers
$8.00/5g
Yield:939-79-7 95%
Reaction Conditions:
with sulfuric acid;nitric acid at 0; for 2 h;
Steps:
38.A
A. 4-Methyl-3-nitrobenzonitrile. Nitric acid (20 mL) was added dropwise to a 0° C. mixture of 4-tolunitrile (11 g, 0.098 mol) in H2SO4 (20 mL) over 1 h. The reaction mixture was stirred at 0° C. for a further hour, then was poured onto crushed ice. The resulting precipitate was collected by filtration, providing the title compound as a white solid (15.2 g, 95%). 1H NMR (500 MHz, CDCl3): 8.27 (d, J=1.6 Hz, 1H), 7.78 (dd, J=8.0, 1.7 Hz, 1H), 7.51 (d, J=8.0 Hz, 1H), 2.69 (s, 3H).
References:
Allison, Brett D.;Hack, Michael D.;Phuong, Victor K.;Rabinowitz, Michael H.;Rosen, Mark D. US2005/38032, 2005, A1 Location in patent:Page/Page column 29
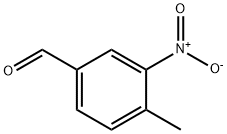
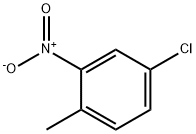
31680-07-6
135 suppliers
$8.00/1g

939-79-7
202 suppliers
$8.00/5g