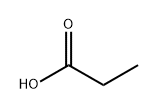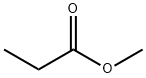
Methyl propionate synthesis
- Product Name:Methyl propionate
- CAS Number:554-12-1
- Molecular formula:C4H8O2
- Molecular Weight:88.11
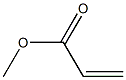
292638-85-8
5 suppliers
inquiry

554-12-1
259 suppliers
$21.00/25mL
Yield:554-12-1 99%
Reaction Conditions:
with [Ir(1-(1′-methylpyrazole)-3-(4′-methylphenyl)triazenide)Cp*Cl];isopropyl alcohol at 90; for 6 h;Catalytic behavior;Inert atmosphere;Glovebox;Reagent/catalyst;
Steps:
2.5. General procedure for transfer hydrogenation reactions monitored by1H NMR and gas chromatography mass spectrometry (GC-MS)
General procedure: In the glovebox, ketone or alken-2-one (6.3×10-5 mol), 1,3,5-trimethylbenzene(2 μL) as internal standard and 0.7 mL of 2-propanol-d8were transferred into a J. Young NMR tube, and a 1H NMR spectrum wasrecorded. The tube was brought back into the glovebox, and precatalyst2 (1.26×10-6 mol in 0.2 mL of 2-propanol-d8) was added. At this point,KOH (0.5 M, 5 μL, 2 eq.) in 2-propanol-d8 was added only to those catalyticreactions performed in presence of base. Outside of the gloveboxthe tube was placed inside an oil bath at 90 °C. The tube was removedfrom the oil bath at different times, and 1H NMR spectra were recordedin order to follow the progress of the reaction. The conversions weredetermined by the integration of either the aromatic protons or the aliphaticprotons of the ketone. The value of the integral for the singlet dueto the aromatic protons of 1,3,5-trimethylbenzene (internal standard)was set to 10 units. In addition, the conversion was also determined byGC-MS analysis in order to follow the deuteration process of the substrates,which is a consequence of using 2-propanol-d8. In addition, thetransfer hydrogenation reactions of some aryl- alkylketones and 5-alken-2-ones were performed as above using the same quantities of reactants(precatalyst 2 or 3 and KOH if needed) in regular 2-propanol.The analytical GC/MS system used was Agilent 7890A GC coupledto 5975C Mass detector Agilent Technologies, equipped with a HP-5MScapillary column (30m×0.25mm×0.25 μm). An AgilentTechnologies 7693 auto sampler was used to inject 1 μL of a solutionsample. The ionization energy was 70 eV with a mass range of 30 to800 m/z. The initial temperature of the column was set at 80 °C, held for2 min, and then a ramp of 10 °C/min to 250 °C. The temperature of theinjector was set at 250 °C and the detector at 230 °C. The flow rate ofthe carrier gas (He) was 1.0 mL/min injected with a gas dilution of1:50. Identification of the individual components was based on comparisonwith the mass spectra library (NIST98).
References:
Medrano-Castillo, Layla J.;Collazo-Flores, Miguel á.;Camarena-Díaz, Juan P.;Correa-Ayala, Erick;Chávez, Daniel;Grotjahn, Douglas B.;Rheingold, Arnold L.;Miranda-Soto, Valentín;Parra-Hake, Miguel [Inorganica Chimica Acta,2020,vol. 507,art. no. 119551]

67-56-1
790 suppliers
$9.00/25ml

74-85-1
113 suppliers
$79.00/11l
201230-82-2
1 suppliers
inquiry

554-12-1
259 suppliers
$21.00/25mL
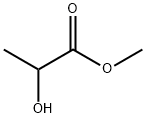
547-64-8
192 suppliers
$15.00/5ml

554-12-1
259 suppliers
$21.00/25mL