Palladium chloride
- CAS No.
- 7647-10-1
- Chemical Name:
- Palladium chloride
- Synonyms
- PALLADIUM(II) CHLORIDE;PALLADIUM DICHLORIDE;Palladium(Ⅱ)chloride;Palladium(II) dichloride;Palladium(II) chloride, 59% Pd;nci-c60184;adium(II) chL;JACS-7647-10-1;Palladiumchlorid;Plladium chloride
- CBNumber:
- CB7222436
- Molecular Formula:
- Cl2Pd
- Molecular Weight:
- 177.33
- MDL Number:
- MFCD00003558
- MOL File:
- 7647-10-1.mol
- MSDS File:
- SDS
| Melting point | 678-680 °C(lit.) |
|---|---|
| Density | 4 g/mL at 25 °C(lit.) |
| vapor pressure | 0Pa at 20℃ |
| storage temp. | Store below +30°C. |
| solubility | 55.6g/l insoluble |
| form | Powder/Solid |
| color | Yellow |
| Specific Gravity | 4 |
| Odor | Odorless |
| PH | 2.15 (30g/l, H2O, 20℃) |
| Water Solubility | Insoluble |
| Decomposition | 200 °C |
| Merck | 14,6990 |
| Stability | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 7647-10-1(CAS DataBase Reference) |
| FDA UNII | N9214IR8N7 |
| EPA Substance Registry System | Palladium dichloride (7647-10-1) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS05,GHS07,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H290-H302-H317-H318-H410 | |||||||||
| Precautionary statements | P234-P273-P280-P301+P312-P302+P352-P305+P351+P338 | |||||||||
| Hazard Codes | C,Xi,T+,T,Xn | |||||||||
| Risk Statements | 34-43-40-28-41-37/38-25-22 | |||||||||
| Safety Statements | 26-36/37/39-45-37/39-28-27-36/37-27/28 | |||||||||
| RIDADR | UN 1789 8/PG 3 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | RT3500000 | |||||||||
| F | 3 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 28439090 | |||||||||
| Toxicity | MLD i.v. in rabbits: 0.0186 g/kg (Orestano) | |||||||||
| NFPA 704 |
|
Palladium chloride price More Price(74)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 921033 | Palladium(II) chloride ≥99.99% trace metals basis | 7647-10-1 | 1G | $139 | 2024-03-01 | Buy |
| Sigma-Aldrich | 921033 | Palladium(II) chloride ≥99.99% trace metals basis | 7647-10-1 | 5G | $546 | 2024-03-01 | Buy |
| Sigma-Aldrich | 921033 | Palladium(II) chloride ≥99.99% trace metals basis | 7647-10-1 | 25G | $2170 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.07110 | Palladium(II) chloride (59% Pd) anhydrous, for synthesis | 7647-10-1 | 500MG | $168 | 2024-03-01 | Buy |
| Sigma-Aldrich | 8.07110 | Palladium(II) chloride (59% Pd) anhydrous, for synthesis | 7647-10-1 | 1G | $310 | 2024-03-01 | Buy |
Palladium chloride Chemical Properties,Uses,Production
Description
Palladium chloride is a commonly used precious metal catalysts, molecular formula is PaCl2, the appearance is brown-red needle-like crystals or powder, easily deliquescence, the relative density is 4.0 (18 ℃), melting point is 500 ℃ (decomposition), soluble in water, ethanol, acetone and hydrogen bromide. Decomposition in ammonia chloride, potassium iodide, ammonia solution, and precipitation of palladium.
[Uses]
(1)used as the analysis reagents, such as determination of trace palladium, mercury, thallium, iodine, etc.
(2) palladium test strips is used to test carbon monoxide.
(3) also used to search for cracks of buried underground gas pipeline cracks, study of agricultural plant resources, preparation of palladium catalyst, electroplating watch parts and photography, and so on.
[Preparation method] by melting palladium dichloride hydrate, make it lost part of chloride to get Palladium chlorine finished products.
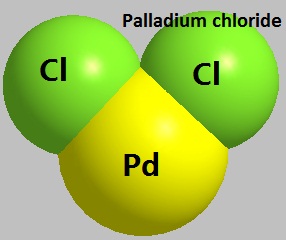
Figure 1 the molecular structure of Palladium chloride.
Chemical Properties
Palladium chloride is a dark brown powder, hygroscopic (absorbs moisture from the air). It is incompatible with acids, aluminium, ammonia, magnesium, nitrates, zinc, heat, thiocyanates, and organic solvents. Thermal decomposition of palladium chloride may release chlorine, hydrogen chloride, and oxides of palladium. It is used as a catalyst, photographic, and electroplating reagent. Palladium and its alloys are used as catalysts in the (petro)chemical and, above all, in the automotive industries. Applications of palladium compounds for electronics and electrical technology include use in metallisation processes (thick film paste), electrical contacts and switching systems, in the synthesis of semiconducting metal-containing polymers in which the polypyrrole backbone has a conformational energy minimum and is nearly planar. Palladium chloride is a stable chemical substance and is incompatible with strong oxidising agents.
Physical properties
l Properties Red rhombohedral crystal; hygroscopic; density 4.0g/cm3; melts at 679°C; dissolves slowly in water; also soluble in ethanol and acetone; dissolves rapidly in hydrochloric acid.
Uses
Palladium(II) chloride(PdCl2) is a dark brown color and is used to coat other metals without the need for electrolysis. It is also used in photography, to make indelible inks, and as a catalyst in analytical chemistry (used to speed up or slow down chemical reactions).
Uses
suzuki reaction
Uses
Palladium chloride is used in photography; toning solutions; electroplating parts of docks and watches; detecting carbon monoxide leaks in buried gas pipes; manufacture of indelible ink; preparation of metal for use as a catalyst; catalyst in jewelry; in dental alloys.
Uses
Palladium dichloride is a starting material for preparing several palladium compounds. It also is used for detection of carbon monoxide. For such detection, a paper is soaked in very dilute solution of PdCl2 which is decolorized by CO, methane and other reducing substances. It also is used in toning and electroplating solutions and in photography for porcelain pictures.
Production Methods
The palladium powder is added to the reactor containing hydrochloric acid, with stirring, passing the air, an oxidation reaction is performed, generating palladium chloride solution, the solution is purified, filtered, concentrated by evaporation, cooling and crystallization, centrifugal separation, and dried to obtain a palladium chloride finished products.
Pd+2HCl+0.5O2→PdCl2+H2O
Definition
ChEBI: Palladium(II) chloride is a palladium coordination entity consisting of palladium(II) bound to two chlorine atoms. It has a role as a catalyst.
Preparation
Palladium dichloride is prepared by dissolving palladium metal in aqua regia or hydrochloric acid in the presence of chlorine. Alternatively, it may be prepared by heating palladium sponge with chlorine gas at 500°C.
Reactions
Palladium dichloride dissolves in HCl forming tetrachloropalladate ion,
PdCl2+2Cl¯→ [PdCl4]2¯
The complex ion catalyzes various types of organic reactions including oxidation of ethylene to acetaldehyde in aqueous solution (the Wacker Process):
PdCl42¯+ C2H4 + H2O → CH3CHO + Pd + 2HCl + 2Cl¯
Palladium dichloride forms polymeric carbonyl complexes when the dry chloride is heated in a stream of carbon monoxide charged with methane vapor. Such complexes include [PdCl2(CO)n] and [PdCl(CO)2]n. The reaction also occurs in aqueous phase resulting in decolorization of the solution.
When H2S is passed through palladium dichloride solution, it yields a brown-black precipitate of palladium monosulfide, PdS.
When heated with sulfur at 450 to 500°C, palladium dichloride forms palladium disulfide, PdS2, a grey-black crystalline compound, insoluble in strong acids but soluble in aqua regia, and which converts to monosulfide, PdS, on heating at 600°C.
When ammonia gas is passed through an aqueous solution of PdCl2, the product is tetrammine palladium(II) chloride, Pd(NH4)2Cl2. The same product also is obtained in dry state by passing ammonia gas over anhydrous PdCl2.
General Description
Dark brown crystals.
Air & Water Reactions
Deliquescent. Water soluble.
Reactivity Profile
Palladium chloride is a weak oxidizing agent. Palladium chloride is reduced in solution by hydrogen or carbon monoxide to metallic palladium. . Decomposed at high temperatures to metallic palladium and chlorine.
Fire Hazard
Flash point data for Palladium chloride are not available. Palladium chloride is probably combustible.
Safety Profile
Poison by intraperitoneat, intravenous, and intratracheal routes. Moderately toxic by ingestion. Experimental reproductive effects. A skin irritant. Questionable carcinogen with experimental carcinogenic data. Human mutation data reported. When heated to decomposition it emits highly toxic fumes of Cl-. See also PALLADIUM
Purification Methods
The anhydrous salt is insoluble in H2O and dissolves in HCl with difficulty. The dihydrate forms red hygroscopic crystals that are readily reduced to Pd. Dissolve it in conc HCl through which dry Cl2 is bubbled. Filter this solution which contains H2PdCl4 and H2PdCl6 and on evaporation it yields a residue of pure PdCl2. [Grube in Handbook of Preparative Inorganic Chemistry (Ed Brauer) Academic Press Vol II p 1582 1965, Mozingo Org Synth Coll Vol III 685 1955.]
Palladium chloride Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1of8
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| XI'AN TIANGUANGYUAN BIOTECH CO., LTD. | +86-029-86333380 18829239519 | sales06@tgybio.com | China | 959 | 58 |
| Shanghai UCHEM Inc. | +862156762820 +86-13564624040 | sales@myuchem.com | China | 6710 | 58 |
| Anhui Ruihan Technology Co., Ltd | +8617756083858 | daisy@anhuiruihan.com | China | 994 | 58 |
| Hebei Jingbo New Material Technology Co., Ltd | +8619931165850 | hbjbtech@163.com | China | 1000 | 58 |
| Hebei Saisier Technology Co., LTD | +86-18400010335 +86-13102810335 | admin@hbsaisier.cn | China | 747 | 58 |
| Springchem New Material Technology Co.,Limited | +86-021-62885108 +8613917661608 | info@spring-chem.com | China | 2068 | 57 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 15928 | 58 |
| Shanghai Bojing Chemical Co.,Ltd. | +86-86-02137122233 +8613795318958 | bj1@bj-chem.com | China | 298 | 55 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Nanjing ChemLin Chemical Industry Co., Ltd. | 025-83697070 | product@chemlin.com.cn | CHINA | 3012 | 60 |
Related articles
- Applications of Palladium chloride
- Palladium chloride is brown to brownish violet powder, red rhombic crystals; hygroscopic. Palladium chloride is readily solubl....
- Nov 15,2019
View Lastest Price from Palladium chloride manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-26 | Palladium Chloride
7647-10-1
|
US $55.00-10.00 / kg | 1kg | 99.9% | 20 tons | Hebei Dangtong Import and export Co LTD | |
 |
2024-04-26 | Palladium chloride
7647-10-1
|
US $33.00-1.00 / kg | 1kg | 99% | 300tons | Hebei Dangtong Import and export Co LTD | |
 |
2024-04-26 | Palladium chloride
7647-10-1
|
US $70.00-65.00 / kilograms | 10kilograms | 99% | 100tons | Hebei Dangtong Import and export Co LTD |
-

- Palladium Chloride
7647-10-1
- US $55.00-10.00 / kg
- 99.9%
- Hebei Dangtong Import and export Co LTD
-

- Palladium chloride
7647-10-1
- US $33.00-1.00 / kg
- 99%
- Hebei Dangtong Import and export Co LTD
-

- Palladium chloride
7647-10-1
- US $70.00-65.00 / kilograms
- 99%
- Hebei Dangtong Import and export Co LTD
7647-10-1(Palladium chloride)Related Search:
1of4