| Identification | More | [Name]
Methyldopa | [CAS]
555-30-6 | [Synonyms]
2-AMINO-3-(3,4-DIHYDROXYPHENYL)-2-METHYL-PROPANOIC ACID
2-METHYL-3-(3,4-DIHYDROXYPHENYL)-L-ALANINE
3-(3,4-DIHYDROXYPHENYL)-2-METHYL-L-ALANINE
3-(3,4-DIHYDROXYPHENYL)-ALPHA-METHYL-L-ALANINE
ALPHA-METHYL-L-BETA-3,4-DIHYDROXYPHENYLALANINE
ALPHA-METHYL-L-DOPA
L-3-(3,4-DIHYDROXYPHENYL)-2-METHYLALANINE
L-(-)-ALPHA-METHYLDOPA
L-ALPHA-METHYL-DOPA
L-METHYLDOPA
MEDOBA
METHYL DOPA
METHYL-L-DOPA
MK-351
3-hydroxy-alpha-methyl-l-tyrosin
3-hydroxy-alpha-methyl-l-tyrosine
3-Hydroxy-methyl-L-tyroxine
4-dihydroxyphenyl)-2-methyl-3-(l-(-)-alanin
aldomet
aldometil | [EINECS(EC#)]
209-089-2 | [Molecular Formula]
C10H13NO4 | [MDL Number]
MFCD00004186 | [Molecular Weight]
211.21 | [MOL File]
555-30-6.mol |
| Chemical Properties | Back Directory | [Melting point ]
≥300 °C
| [Boiling point ]
350.89°C (rough estimate) | [density ]
1.2545 (rough estimate) | [refractive index ]
-14 ° (C=1, H2O) | [storage temp. ]
Sealed in dry,2-8°C | [solubility ]
Soluble to 75 mM in DMSO | [form ]
powder to crystal | [pka]
2.28±0.26(Predicted) | [color ]
White to Almost white | [Water Solubility ]
10g/L(temperature not stated) | [Merck ]
6055 | [BCS Class]
3 | [InChI]
InChI=1/C10H13NO4/c1-10(11,9(14)15)5-6-2-3-7(12)8(13)4-6/h2-4,12-13H,5,11H2,1H3,(H,14,15)/t10-/s3 | [InChIKey]
CJCSPKMFHVPWAR-JQHDBZEONA-N | [SMILES]
C(C1C=CC(O)=C(O)C=1)[C@@](N)(C)C(=O)O |&1:9,r| | [CAS DataBase Reference]
555-30-6(CAS DataBase Reference) | [EPA Substance Registry System]
555-30-6(EPA Substance) |
| Safety Data | Back Directory | [WGK Germany ]
3
| [RTECS ]
YP2860000
| [F ]
10-23 | [TSCA ]
TSCA listed | [HS Code ]
29181990 | [Safety Profile]
Poison by
intraperitoneal route. Moderately toxic by
ingestion and intravenous routes. Human
systemic effects by ingestion: fasciculations,
hallucinations, distorted perceptions,
tremors, allergic dermatitis, necrotic
gastrointestinal changes. An experimental
teratogen. Human reproductive effects:
menstrual cycle changes or disorders, effects
on newborn including abnormal neonatal
measures and growth statistics, biochemical
and metabolic changes. Experimental reproductive effects. Mutation data
reported. When heated to decomposition it
emits toxic fumes of NOx | [Hazardous Substances Data]
555-30-6(Hazardous Substances Data) | [Toxicity]
LD50 oral in rabbit: 713mg/kg |
| Hazard Information | Back Directory | [General Description]
Colorless or almost colorless crystals or white to yellowish-white fine powder. Almost tasteless. In the sesquihydrate form. pH (saturated aqueous solution) about 5.0. | [Reactivity Profile]
METHYL DOPA(555-30-6) undergoes catalytic oxygenation in the presence of magnesium, cupric, cobalt, nickel and ferric ions . A weakly acidic amino acid. | [Air & Water Reactions]
Very hygroscopic. Slightly water soluble. May be sensitive to prolonged exposure to air and light. The stability of aqueous solutions is markedly dependent on pH, oxygen and the amount of initial reactant. Aqueous solutions are stable for up to 50 hours in acid and neutral pH (6.2). At pH 8.0, decomposition products are formed in 3 to 5 hours. Solutions develop a red tint that becomes progressively darker (eventually forming a black precipitate). | [Fire Hazard]
Flash point data for this chemical are not available; however, METHYL DOPA is probably combustible. | [Description]
Methyldopa is an α-methoxylated derivative of levodopa that exhibits hypotensive action
by reducing overall peripheral vascular resistance and reducing heart work.
Antihypertensive action of methyldopa consists of the biotransformation of methyldopa
into methylnoradrenaline (methylnorepinephrine), which acts as a “pseudo neurotransmitter.”
The current, universally accepted point of view is that the action of methyldopa is carried
out through the CNS, where methylnorepinephrine, a powerful stimulant of
α-adrenergic receptors of the medulla, inhibits the vasomotor center. | [Originator]
Aldometil,MSD,W. Germany,1962 | [Uses]
Antihypertensor;L-aromatic aminoacid decarboxylase inhibitor | [Uses]
It is prescribed for arterial hypertension and hypertensive crises. | [Uses]
vitamin, coenzyme B12 | [Definition]
ChEBI: A derivative of L-tyrosine having a methyl group at the alpha-position and an additional hydroxy group at the 3-position on the phenyl ring. | [Manufacturing Process]
The dl-α-methyl-3,4-dihydroxyphenylalanine may be made as described in US
Patent 2,868,818. Five-tenths of a gram of 3-hydroxy-4-
methoxyphenylalaninewas dissolved in 20 ml of concentrated hydrochloric
acid, the solution saturated with hydrogen chloride and heated in a sealed
tube at 150°C for 2 hours. The dark reaction mixture was concentrated to
dryness in vacuo, excess acid removed by flushing several times with ethanol.
On dissolving the dark residue in a minimum amount of water and adjusting
the clarified solution to pH 6.5 with ammonium hydroxide the compound
separated in fine crystals which were filtered, washed with alcohol and ether.
The crystalline product had a MP of 299.5 to 300°C with decomposition.
Then, as described in US Patent 3,158,648, the optical isomers may be
resolved as follows. 37 g of racemic α-methyl-3,4-dihydroxyphenylalanine are
slurried at 35°C in 100 cc of 1.0 N hydrochloric acid. The excess solids are
filtered leaving a saturated solution containing 34.6 g of racemic amino acid of
which about 61% is present as the hydrochloride. The solution is then seeded
at 35°C with 7 g of hydrated L-α-methyl-3,4-dihydroxyphenylalanine (6.2 g of
anhydrous material). The mixture is then cooled to 20°C in 30 minutes and
aged one hour at 20°C. The separated material is isolated by filtration,
washed twice with 10 cc of cold water and dried in vacuo. The yield of product
is 14.1 g of L-α-methyl-3,4-dihydroxyphenylalanine in the form of a
sesquihydrate of 100% purity as determined by the rotation of the copper
complex. | [Therapeutic Function]
Antihypertensive | [Biological Functions]
The spectrum of activity of α-methyldopa (Aldomet)
lies between those of the more potent agents, such as
guanethidine, and the milder antihypertensives, such as
reserpine. α-Methyldopa is a structural analogue of dihydroxyphenylalanine
(dopa) and differs from dopa
only by the presence of a methyl group on the �-carbon
of the side chain. | [Biological Activity]
L-aromatic amino acid decarboxylase inhibitor. Antihypertensive. | [Mechanism of action]
A number of theories have been put forward to account
for the hypotensive action of α-methyldopa. Current
evidence suggests that for α-methyldopa to be an antihypertensive
agent, it must be converted to α-methylnorepinephrine;
however, its site of action appears to be
in the brain rather than in the periphery. Systemically administered
α-methyldopa rapidly enters the brain,
where it accumulates in noradrenergic nerves, is converted
to α-methylnorepinephrine, and is released.
Released α-methylnorepinephrine activates CNS α-
adrenoceptors whose function is to decrease sympathetic
outflow. Why α-methylnorepinephrine decreases sympathetic
outflow more effectively than does the naturally
occurring transmitter is not entirely clear. | [Pharmacokinetics]
The oral bioavailability of methyldopa ranges from 20 to 50% and varies among individuals. Optimum blood pressure
response occurs in 12 to 24 hours in most patients. After withdrawal of the drug, blood pressure returns to
pretreatment levels within 24 to 48 hours. Methyldopa and its metabolites are weakly bound to plasma proteins.
Although 95% of a dose of methyldopa is eliminated in hypertensive patients with normal renal function, with a plasma
half-life of approximately 2 hours, in patients with impaired renal function the half-life is doubled to approximately 3 to
4 hours, with about 50% of it excreted. Orally administered methyldopa undergoes presystemic first-pass metabolism in
the gastrointestinal (GI) tract to its 3-O-monosulfate metabolite. Sulfate conjugation occurs to a greater extent when
the drug is given orally than when it is given intravenously (IV). Its rate of sulfate conjugation is decreased in patients
with renal insufficiency. Methyldopa is excreted in urine as its mono-O-sulfate conjugate. Any peripherally
decarboxylated α-methylnorepinephrine is metabolized by catecho-o-methyltransferase (COMT) and monoamine
oxidase (MAO).
Methyldopate is slowly hydrolyzed in the body to form methyldopa. The hypotensive effect of IV methyldopate begins in
4 to 6 hours and lasts 10 to 16 hours. | [Pharmacology]
The primary hemodynamic alteration responsible for
the hypotensive effects of α-methyldopa remains in dispute.
When the patient is supine, the reduction in blood
pressure produced by α-methyldopa correlates best
with a decrease in peripheral vascular resistance, cardiac
output being only slightly reduced. When the patient
is upright, the fall in blood pressure corresponds
more closely with a reduced cardiac output.
An important aspect of α-methyldopa’s hemodynamic
effects is that renal blood flow and glomerular filtration
rate are not reduced. As occurs with most sympathetic
depressant drugs and vasodilators, long-term
therapy with α-methyldopa leads to fluid retention,
edema formation, and plasma volume expansion.While
data conflict somewhat, it is generally thought that �-
methyldopa suppresses plasma renin activity. | [Clinical Use]
α-Methyldopa is not generally believed to be suitable
for monotherapy of primary hypertension. Because
plasma volume increases as the duration of α-methyldopa
therapy is extended, the drug should be used in
conjunction with a diuretic; this will produce a significantly
greater fall in blood pressure than would occur
with either drug used alone. Because α-methyldopa lowers
blood pressure without compromising either renal
blood flow or the glomerular filtration rate, it is particularly
valuable in hypertension complicated by renal disease.
However, if end-stage renal failure accompanies severe
hypertension,α-methyldopa may not be effective.
The presence of α-methyldopa and its metabolites
in the urine reduces the diagnostic value of urinary catecholamine
measurements as an indicator of pheochromocytoma,
since these substances interfere with the fluorescence
assay for catecholamines. | [Side effects]
The most commonly encountered side effects of α-
methyldopa are sedation and drowsiness.These CNS effects
are probably the result of reductions in brain catecholamine
levels. Other side effects, also typical of
sympathetic depression, are dry mouth, nasal congestion,
orthostatic hypertension, and impotence.
Autoimmune reactions associated with α-methyldopa
treatment include thrombocytopenia and leukopenia.
Since a few cases of an α-methyldopa–induced hepatitis
have occurred, the drug is contraindicated in
patients with active hepatic disease. Flulike symptoms
also are known to occur. | [Synthesis]
Methyldopa, (-)-3-(3,4-dihydroxyphenyl)-2-methylalanine (22.2.5), is
synthesized by a few methods that are only slightly different. The first method is from 3,4-
dimethoxyphenylacetone, which undergoes a Strecker¨CZelinski reaction using
potassium cyanide and ammonium carbonate, to give 4-methyl-4-(3,4-dimethoxybenzylhydantoine
(22.3.3), which is further hydrolyzed in the presence of barium hydroxide
to give (�)-3-(3,4-dimethoxyphenyl)-2-methylalanine (22.3.4). This undergoes
acetylation at the amino group, and the racemic mixture is then separated using
(-)-1-phenylethylamine. The isolated isomer is hydrolyzed using hydrobromic acid, which
simultaneously removes the methoxy- and acetyl groups to give the desired
(-)-3-(3,4-dihydroxyphenyl)-2-methylalanine (22.3.5) [8¨C10]. Alternative syntheses have
been proposed.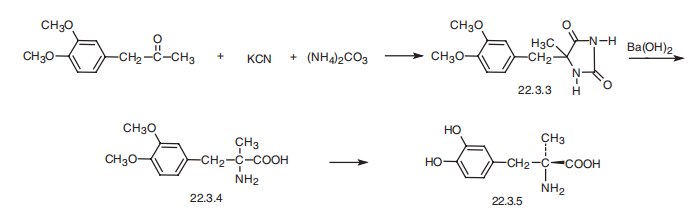
| [Drug interactions]
Potentially hazardous interactions with other drugs
Anaesthetics: enhanced hypotensive effect.
Antidepressants: avoid concomitant use with
MAOIs.
Lithium: neurotoxicity (without increased plasma lithium concentrations).
Salbutamol: acute hypotension reported with
salbutamol infusions. | [Metabolism]
Approximately 50% of an orally administered dose of
α-methyldopa is absorbed from the gastrointestinal
tract. Both peak plasma drug levels and maximal blood
pressure–lowering effects are observed 2 to 6 hours after
oral administration. A considerable amount of unchanged
α-methyldopa and several conjugated and decarboxylated
metabolites can be found in the urine. | [storage]
Room temperature | [Purification Methods]
Recrystallise methyldopa from H2O. [Reinhold et al. J Org Chem 33 1209 1968.] The L-isomer forms a sesquihydrate from H2O m 302-304o (dec), and the anhydrous crystals are hygroscopic,[�] 23D -4.0o (c 1, 0.1N HCl), [�]546 +154.5o (c 5, CuSO4 solution). It has max at 281nm ( 2780). Its solubility in H2O at 25o is ~10mg/mL and the pH of an aqueous solution is ~5.0. It is insoluble in most organic solvents. [Stein et al. J Am Chem Soc 77 700 1955, Beilstein 4 IV 2505.] |
|
|