| Identification | More | [Name]
Aniline | [CAS]
62-53-3 | [Synonyms]
AKOS BBS-00003680
AMINOBENZENE
ANILINE
ANILINE OIL
BENZENAMINE
BENZENEAMINE
PHENYLAMINE
ai3-03053
amino-benzen
Aminophen
Anilin
anilin(czech)
Anilina
anilina(italian,polish)
Aniline reagent
aniline(andhomologs)
Aniline(benzenamine)
aniline15
anilineandhomologues
Anyvim | [EINECS(EC#)]
200-539-3 | [Molecular Formula]
C6H7N | [MDL Number]
MFCD00007629 | [Molecular Weight]
93.13 | [MOL File]
62-53-3.mol |
| Chemical Properties | Back Directory | [Appearance]
Aniline is a clear, colorless, oily liquid that darkens
on exposure to light; with a characteristic amine-like
odor. | [Melting point ]
-6 °C (lit.) | [Boiling point ]
184 °C (lit.) | [density ]
1.022 g/mL at 25 °C(lit.)
| [vapor density ]
3.22 (185 °C, vs air)
| [vapor pressure ]
0.7 mm Hg ( 25 °C)
| [refractive index ]
n20/D 1.586(lit.)
| [Fp ]
76 °C
| [storage temp. ]
2-8°C | [solubility ]
water: soluble | [form ]
Liquid | [pka]
4.63(at 25℃) | [color ]
APHA: ≤250 | [Specific Gravity]
1.021 | [Odor]
Sweet, amine-like odor detectable at 0.6 to 10 ppm | [PH]
8.8 (36g/l, H2O, 20℃) | [PH Range]
8.1 | [Relative polarity]
0.42 | [Stability:]
Stable. Incompatible with oxidizing agents, bases, acids, iron and iron salts, zinc, aluminium. Light sensitive. Combustible. | [explosive limit]
1.2-11%(V) | [Water Solubility ]
36 g/L (20 ºC) | [Merck ]
14,659 | [BRN ]
605631 | [Henry's Law Constant]
1.91 at 25 °C (thermodynamic method-GC/UV spectrophotometry, Altschuh et al., 1999) | [Dielectric constant]
7.8(0℃) | [Exposure limits]
TLV-TWA skin 2 ppm (~8 mg/m3) (ACGIH),
5 ppm (~19 mg/m3) (MSHA, OSHA, and
NIOSH); IDLH 100 ppm (NIOSH). | [Major Application]
environmental | [InChI]
1S/C6H7N/c7-6-4-2-1-3-5-6/h1-5H,7H2 | [InChIKey]
PAYRUJLWNCNPSJ-UHFFFAOYSA-N | [SMILES]
Nc1ccccc1 | [LogP]
0.900 | [Surface tension]
47.9mN/m at 298.15K | [Uses]
A thin, colorless oil prepared by reducing benzene with iron
filings in the presence of hydrochloric or acetic acid and then
separating the aniline formed by distillation. It is slightly
soluble in water but dissolves easily in alcohol, ether, and
benzene. Aniline is the base for many dyes used to increase
the sensitivity of emulsions. | [CAS DataBase Reference]
62-53-3(CAS DataBase Reference) | [IARC]
2A (Vol. 27, Sup 7, 127) | [NIST Chemistry Reference]
Aniline(62-53-3) | [Storage Precautions]
Light sensitive;Store under nitrogen | [EPA Substance Registry System]
62-53-3(EPA Substance) |
| Hazard Information | Back Directory | [Chemical Properties]
Aniline was fi rst isolated from the destructive distillation of indigo in 1826 by Otto
Unverdorben. Aniline is oily and, although colorless, it slowly oxidizes and turns into
a kind of resin in air, giving the sample a red-brown tint. At room temperature, aniline,
the simplest aromatic amine, is a clear to slightly yellow, oily liquid that darkens to a
brown color on exposure to air. Like most volatile amines, it possesses the somewhat
unpleasant odor of rotten fi sh and also has a burning aromatic taste. It has a low vapor
pressure at room temperature and ignites readily, burning with a smoky flame. It does
not readily evaporate at room temperature. Aniline is slightly soluble in water and
mixes readily with most organic solvents. It is synthesized by catalytic hydrogenation
of nitrobenzene or by ammonolysis of phenol. Aniline is incompatible with strong
acids, strong oxidizers, albumin, and solutions of iron, zinc, aluminum, toluene diisocyanate, and alkalis. It ignites spontaneously in the presence of red fuming nitric acid,
and with sodium.
Originally, the great commercial value of aniline was due to the readiness with
which it yields, directly or indirectly, valuable dyestuffs. Currently, the largest market
for aniline is in the preparation of methylene diphenyl diisocyanate (MDI), some 85%
of aniline serving this market. In fact, in industry, aniline is an initiator or intermediary in the synthesis of aniline being used as a precursor to more complex chemicals. It
is the starting material for many dyestuffs, known as aniline dyes. Its main application
is in the manufacture of polyurethane foam, and a wide variety of products, such as
MDI, agricultural chemicals, synthetic dyes, antioxidants, stabilizers for the rubber industry, varnishes, explosives, analgesics, and hydroquinone for photographic developing, and as an octane booster in gasoline. Aniline has also been detected in tobacco
smoke and exposures to aniline have been reported among workers in related industrial workplaces, hazardous waste sites, and the general population through food and
drinking water. | [General Description]
A yellowish to brownish oily liquid with a musty fishy odor. Melting point-6°C; boiling point 184°C; flash point 158°F. Denser than water (8.5 lb/gal) and slightly soluble in water. Vapors heavier than air. Toxic by skin absorption and inhalation. Produces toxic oxides of nitrogen during combustion. Used to manufacture other chemicals, especially dyes, photographic chemicals, agricultural chemicals and others. | [Reactivity Profile]
ANILINE(62-53-3) is a heat sensitive base. Combines with acids to form salts. Dissolves alkali metals or alkaline earth metals with evolution of hydrogen. Incompatible with albumin, solutions of iron, zinc and aluminum, and acids. Couples readily with phenols and aromatic amines. Easily acylated and alkylated. Corrosive to copper and copper alloys. Can react vigorously with oxidizing materials (including perchloric acid, fuming nitric acid, sodium peroxide and ozone). Reacts violently with BCl3. Mixtures with toluene diisocyanate may ignite. Undergoes explosive reactions with benzenediazonium-2-carboxylate, dibenzoyl peroxide, fluorine nitrate, nitrosyl perchlorate, peroxodisulfuric acid and tetranitromethane. Violent reactions may occur with peroxyformic acid, diisopropyl peroxydicarbonate, fluorine, trichloronitromethane (293° F), acetic anhydride, chlorosulfonic acid, hexachloromelamine, (HNO3 + N2O4 + H2SO4), (nitrobenzene + glycerin), oleum, (HCHO + HClO4), perchromates, K2O2, beta-propiolactone, AgClO4, Na2O2, H2SO4, trichloromelamine, acids, FO3Cl, diisopropyl peroxy-dicarbonate, n-haloimides and trichloronitromethane. Ignites on contact with sodium peroxide + water. Forms heat or shock sensitive explosive mixtures with anilinium chloride (detonates at 464° F/7.6 bar), nitromethane, hydrogen peroxide, 1-chloro-2,3-epoxypropane and peroxomonosulfuric acid. Reacts with perchloryl fluoride form explosive products. . | [Air & Water Reactions]
Darkens on exposure to air and light. Polymerizes slowly to a resinous mass on exposure to air and light. Slightly soluble in water. | [Hazard]
An allergen. Toxic if absorbed through the
skin. Combustible. Skin irritant. Questionable car-
cinogen.
| [Health Hazard]
ANILINE is classified as very toxic. Probable oral lethal dose in humans is 50-500 mg/kg for a 150 lb. person. Aniline poisoning is characterized by methemoglobin formation in the blood and resulting cyanosis or blue skin. The formation of methemoglobin interferes with the oxygen-carrying capacity of the blood. The approximate minimum lethal dose for a 150 lb. human is 10 grams. Serious poisoning may result from ingestion of 0.25 mL. People at special risk include individuals with glucose-6-phosphate-dehydrogenase deficiency and those with liver and kidney disorders, blood diseases, or a history of alcoholism. | [Health Hazard]
Exposures to aniline on inhalation, ingestion and/or through skin contact cause adverse
health effects. Exposures to liquid aniline cause mild irritation to the skin and eyes.
Aniline is a blood toxin and its absorption through the skin and by inhalation of its
vapor results in systemic toxicity, damage to the kidney, liver, bone marrow and of methemoglobinemia. The symptoms of poisoning include, but are not limited to, drowsiness, dizziness, severe headache, nausea, tiredness, bluish discoloration of the lips and
tongue, loss of appetite, irregular heart beat, mental confusion, and shock. A prolonged
period of exposure to the vapor results in respiratory paralysis, convulsions, coma, and
death.
| [Potential Exposure]
Aniline is widely used as an intermediate
in the synthesis of dyestuffs. It is also used in the
manufacture of rubber accelerators and antioxidants, pharmaceuticals,
marking inks; tetryl, optical whitening agents;
photographic developers; resins, varnishes, perfumes, shoe
polishes, and many organic chemicals. | [Fire Hazard]
Combustion can produce toxic fumes including nitrogen oxides and carbon monoxide. Aniline vapor forms explosive mixtures with air. ANILINE is incompatible with strong oxidizers and strong acids and a number of other materials. Avoid heating. Hazardous polymerization may occur. Polymerizes to a resinous mass. | [First aid]
If this chemical gets into the eyes, remove any
contact lenses at once and irrigate immediately for at least
15 minutes, occasionally lifting upper and lower lids. Seek
medical attention immediately. If this chemical contacts the
skin, remove contaminated clothing and wash immediately
with soap and water. Seek medical attention immediately.
If this chemical has been inhaled, remove from exposure,
begin rescue breathing (using universal precautions, including
resuscitation mask) if breathing has stopped and CPR if
heart action has stopped. Transfer promptly to a medical
facility. When this chemical has been swallowed, get medical
attention. Give large quantities of water and induce
vomiting. Do not make an unconscious person vomit.
| [Shipping]
UN1547 Aniline, Hazard Class: 6.1; Labels: 6.1-
Poisonous materials. UN1548 Aniline hydrochloride,
Hazard Class: 6.1; Labels: 6.1-Poisonous materials. | [Incompatibilities]
May form explosive mixture with air.
Unless inhibited (usually methanol), aniline is readily able
to polymerize. Fires and explosions may result from contact
with halogens, strong acids; oxidizers, strong base organic
anhydrides; acetic anhydride, isocyanates, aldehydes,
sodium peroxide. Strong reaction with toluene diisocyanate.
Reacts with alkali metals and alkali earth metals. Attacks
some plastics, rubber and coatings; copper and copper
alloys. | [Waste Disposal]
Consult with environmental
regulatory agencies for guidance on acceptable disposal
practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing
storage, transportation, treatment, and waste disposal.
Incineration with provision for nitrogen oxides removal from
flue gases by scrubber, catalytic or thermal device. | [Physical properties]
Colorless, oily liquid with a faint ammonia-like odor and burning taste. Gradually becomes yellow
to reddish-brown on exposure to air or light. The lower and upper odor thresholds are 2 and 128
ppm, respectively (quoted, Keith and Walters, 1992). An odor threshold of 1.0 ppmv was reported
by Leonardos et al. (1969). | [Definition]
ChEBI: A primary arylamine in which an amino functional group is substituted for one of the benzene hydrogens. | [Production Methods]
Aniline was obtained in 1826 by Unverdorben from distillation of indigo and was given the name aniline in 1841 by Fritzsche (Windholz et al 1983). The chemical was manufactured in the U. S. by the Bechamp reaction involving reduction of nitrobenzene in the presence of either copper/silica or hydrochloric acid/ferrous chloride catalysts; but in 1966, amination of chlorobenzene with ammonia was introduced (IARC 1982; Northcott 1978). Currently, aniline is produced in the U.S., several European countries and Japan by the catalytic hydrogenation of nitrobenzene in either the vapor phase or solvent system. This chemical is also produced by reacting phenol with ammonia (HSDB 1989). Production in 1982 amounted to 331,000 tons (HSDB 1989). | [Synthesis Reference(s)]
Chemical and Pharmaceutical Bulletin, 29, p. 1159, 1981 DOI: 10.1248/cpb.29.1159
The Journal of Organic Chemistry, 58, p. 5620, 1993 DOI: 10.1021/jo00073a018 | [Flammability and Explosibility]
Aniline is a combustible liquid (NFPA rating = 2). Smoke from a fire involving
aniline may contain toxic nitrogen oxides and aniline vapor. Toxic aniline vapors are
given off at high temperatures and form explosive mixtures in air. Carbon dioxide or
dry chemical extinguishers should be used to fight aniline fires. | [Chemical Reactivity]
Reactivity with Water No reaction; Reactivity with Common Materials: No reaction; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Flush with water and rinse with dilute acetic acid; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent. | [Industrial uses]
Aniline, the simplest primary aromatic amine, consists of a nitrogen atom with two
attached hydrogen atoms affixed to a benzene ring. This aromatic amine is a
weaker base than the aliphatic amines but aniline does undergo many of the same
reactions in the realm of synthetic chemistry. Aniline is used to prepare agricultural
chemicals, antioxidants, fungicides, herbicides, isocyanates, and other
commercially important chemicals.
Aniline is used as a chemical intermediate to prepare isocyanates for making polyurethanes,
antioxidants, and vulcanization accelerators, as well as in the manufacture
of agricultural fungicides, herbicides and insecticides and in the preparation
of certain dyes. | [Biochem/physiol Actions]
The acute toxicity of aniline involves its activation in vivo to 4-hydroxyaniline and the formation of adducts with hemoglobin. In erythrocytes, this is associated with the release of iron and the accumulation of methemoglobin and the development of hemolytic anemia and inflammation of the spleen. Tumor formation is often observed in the spleen on prolonged administration. | [Toxicology]
Aniline, a weakly alkaline liquid, is readily absorbed
into the circulation after oral ingestion,
inhalation and dermal contact. In human volunteers,
more than 90 % of the inhaled aniline vapors
(5 – 30 mg/m3) were absorbed in the state of
rest . The percutaneous uptake from the
vapor phase accounted for 25 – 30 % of the total
incorporation in normally dressed individuals at
25 ?C and 35 % relative air humidity (estimated
absorption rate: 0.2 – 0.4 μg cm?2h?1), but increased
by 21 and 29 % when the temperature
was elevated by 5 ?Cand the humidity from 35 to
75 %, respectively . Likewise, when applied
as liquid to human skin from a drained gauze
(concentration 10 mg/cm2), skin absorption of
aniline was between 0.2 and 0.7 mg cm?2h?1
but could reach up to 3.5 mg cm?2h?1 on highly
moistened skin , also temperature appeared
to be a factor.
Aniline undergoes rapid oxidation, mainly in
the liver, but also in other organs like the intestine
and erythrocytes. Three primary transformation
reactions compete with each other and are expressed
to varying degree in different species
and individuals:
1) N-Hydroxylation
2) (Ring) hydroxylation
3) N-Acetylation followed by p-(ring) hydroxylation
In secondary steps, the hydroxyl intermediates
are rapidly conjugated, largely to sulfate and
glucuronic acid and excreted, mainly in the urine
. In humans, the half-life of aniline is ca.
3.5 h .
The primary conversion products, mainly
phenylhydroxylamine and p-aminophenol as
well as their oxidized forms nitrosobenzene
and p-iminoquinone, resulting from reactions
1 and 2, are regarded as toxification steps
to biologically active compounds (see below),
whereas N-acetylation may be considered as
a detoxification step, which is followed by phydroxylation
to N-acetyl-p-aminophenol. NAcetyl
transferase is congenitally expressed to
varying extent in humans (“strong and weak
acetylators”; see below); this is a reason for different
individual susceptibilities.
Certain metabolites, such as nitrosobenzene,
are coupled to thiols, especially glutathione; the
quantities of aniline-protein conjugates, especially
aniline-Hb adducts in blood, are diagnostic
tools for the estimation of aniline exposure
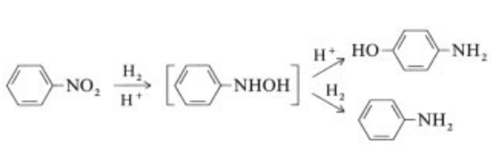
and body burden . | [Synthesis]
The highly exothermic catalytic hydrogenation
(ΔH =?544 kJ/mol at 200 ?C) of nitrobenzene
is performed both in the vapor and in the liquid
phase in commercially used processes .
 | [Carcinogenicity]
The IARC has classified aniline as a Group 3 carcinogen,
that is, not classifiable as to its carcinogenicity. However,
NIOSH has determined that there is sufficient evidence
to recommend that OSHA require labeling this substance a
potential occupational carcinogen. This position followed an
evaluation of a high-dose feeding study of aniline hydrochloride in F344 rats and B6C3F1 mice (3000 or
6000 ppm and 6000 or 12,000 ppm, respectively). The test
was negative in both sexes of mice; however, hemangiosarcomas
of the spleen and combined incidence of fibrosarcomas
and sarcomas of the spleen were statistically significant
in the male rats; the number of female rats having fibrosarcomas
of the spleen was also significant. | [Source]
Detected in distilled water-soluble fractions of regular gasoline (87 octane) and Gasohol
at concentrations of 0.55 and 0.20 mg/L, respectively (Potter, 1996). Aniline was also detected in
82% of 65 gasoline (regular and premium) samples (62 from Switzerland, 3 from Boston, MA). At
25 °C, concentrations ranged from 70 to 16,000 μg/L in gasoline and 20 to 3,800 μg/L in watersoluble
fractions. Average concentrations were 5.8 mg/L in gasoline and 1.4 mg/L in watersoluble
fractions (Schmidt et al., 2002).
Based on laboratory analysis of 7 coal tar samples, aniline concentrations ranged from ND to 13
ppm (EPRI, 1990).
Aniline in the environment may originate from the anaerobic biodegradation of nitrobenzene
(Razo-Flores et al., 1999). | [Environmental Fate]
Biological. Under anaerobic conditions using a sewage inoculum, 10% of the aniline present
degraded to acetanilide and 2-methylquinoline (Hallas and Alexander, 1983). In a 56-d
experiment, [14C]aniline applied to soil-water suspensions under aerobic and anaerobic conditions
gave 14CO2 yields of 26.5 and 11.9%, respectively (Scheunert et al., 1987). A bacterial culture
isolated from the Oconee River in North Georgia degraded aniline to the intermediate catechol
(Paris and Wolfe, 1987). Aniline was mineralized by a soil inoculum in 4 d (Alexander and
Lustigman, 1966).
Soil. A reversible equilibrium is quickly established when aniline covalently bonds with
humates in soils forming imine linkages. These quinoidal structures may oxidize to give nitrogensubstituted
quinoid rings. The average second-order rate constant for this reaction in a pH 7 buffer
at 30 °C is 9.47 x 10-5 L/g?h (Parris, 1980). In sterile soil, aniline partially degraded to azobenzene,
phenazine, formanilide, and acetanilide and the tentatively identified compounds nitrobenzene and
p-benzoquinone (Pillai et al., 1982).
Surface Water. Aniline degraded in pond water containing sewage sludge to catechol, which
then degrades to carbon dioxide. Intermediate compounds identified in minor degradative
pathways include acetanilide, phenylhydroxylamine, cis,cis-muconic acid, β-ketoadipic acid,
levulinic acid, and succinic acid (Lyons et al., 1984).
Photolytic. A carbon dioxide yield of 46.5% was achieved when aniline adsorbed on silica gel
was irradiated with light (λ >290 nm) for 17 h (Freitag et al., 1985). Products identified from the
gas-phase reaction of ozone with aniline in synthetic air at 23 °C were nitrobenzene, formic acid,
hydrogen peroxide, and a nitrated salt having the formula: [C6H5NH3]+NO3
- (Atnagel and
Himmelreich, 1976). A second-order rate constant of 6.0 x 10-11 cm3/molecule?sec at 26 °C was
reported for the vapor-phase reaction of aniline and OH radicals in air at room temperature
(Atkinson, 1985).
Chemical/Physical. Alkali or alkaline earth metals dissolve in aniline with hydrogen evolution
and the formation of anilides (Windholz et al., 1983). Laha and Luthy (1990) investigated the
redox reaction between aniline and a synthetic manganese dioxide in aqueous suspensions at the
pH range 3.7–6.5. They postulated that aniline undergoes oxidation by loss of one electron
forming cation radicals. These radicals may undergo head-to-tail, tail-to-tail, and head-to-head couplings forming 4-aminophenylamine, benzidine, and hydrazobenzene, respectively. These
compounds were additionally oxidized, in particular, hydrazobenzene to azobenzene at pH 4 (Laha
and Luthy, 1990). | [Metabolism]
Aniline is absorbed from the skin and the gastrointestinal tract (BaranowskaDutkeiwicz 1982). It is excreted primarily in the urine of treated rabbits with only a small fraction (2%) of the administered dose excreted in the feces (Kao et al 1978; Parke 1960) and none in the expired air. Urinary metabolites of aniline include P-aminophenol, O-aminophenol, m-aminophenol, aniline-N-glucuronide, phenylsulfonic acid and acetanilide (Parke, 1960). Aminophenyl- and acetylaminophenyl-mercapturic acids also have been detected in the urine of rats and rabbits (IARC 1982). Excretion of aniline conjugates of P-aminophenol have been observed in human urine (Williams 1959) and urinary excretion of these conjugates has been found to reflect the extent of absorption of aniline vapor through the skin and respiratory tract (Kao et al 1978; Piotrowski 1972). The methemoglobinemia produced in humans by aniline is believed to result from its N-hydroxylation (IARC 1982). Aniline also is a weak inducer of hepatic microsomal enzymes. Subcutaneous injections of 5 mg/kg body weight for 30 days to rats impaired aniline metabolism in vivo but it increased its in vitro metabolism to p-aminophenol (Wisniewska-Knypl and Jablonska 1975; Wisniewska-Knypl et al 1975). Low protein diets decreased hepatic aniline hydroxylation in the rat (Kato et al 1968). Saturated fat increased aniline metabolism by rat liver independent of chemical composition of the fat used (Caster et al 1970). Highest initial concentrations of aniline derived radioactivity were found in blood, liver, kidney, bladder, and gastrointestinal tract of rat, given labelled compound i.v. After 0.5 h and 6 h, radioactivity concentrated in the stomach and jejunum and subsequently absorbed from the intestine indicating the presence of an enterogastric cycle in rats. Aniline was the predominant compound in the gastric contents of treated animals and acetanilide is the major metabolite found in the jejunal contents (Irons et al 1980). | [Purification Methods]
Aniline is hygroscopic. It can be dried with KOH or CaH2, and distilled under reduced pressure. Treatment with stannous chloride removes sulfur-containing impurities, reducing the tendency to become coloured by aerial oxidation. It can be crystallised from Et2O at low temperatures. More extensive purifications involve preparation of derivatives, such as the double salt of aniline hydrochloride and cuprous chloride or zinc chloride, or N-acetylaniline (m 114o) which can be recrystallised from water. Redistilled aniline is dropped slowly into a strong aqueous solution ofrecrystallised oxalic acid. Aniline oxalate (m 174-175o) is filtered off, washed several times with water and recrystallised three times from 95% EtOH. Treatment with saturated Na2CO3 solution regenerated aniline which was distilled from the solution, dried and redistilled under reduced pressure [Knowles Ind Eng Chem 12 881 1920]. After refluxing with 10% acetone for 10hours, aniline is acidified with HCl (Congo Red as indicator) and extracted with Et2O until colourless. The hydrochloride is purified by repeated crystallisation before aniline is liberated by addition of alkali, then dried with solid KOH, and distilled. The product is sulfur-free and remains colourless in air [Hantzsch & Freese Chem Ber 27 2529, 2966 1894]. Non-basic materials, including nitro compounds, are removed from aniline in 40% H2SO4 by passing steam through the solution for 1hour. Pellets of KOH are then added to liberate the aniline which is steam distilled, dried with KOH, distilled twice from zinc dust at 20mm, dried with freshly prepared BaO, and finally distilled from BaO in an all-glass apparatus [Few & Smith J Chem Soc 753 1949]. Aniline is absorbed through skin and is TOXIC.[Beilstein 12 IV 223.] | [Toxicity evaluation]
Although largely a synthetic chemical, aniline is produced from
the burning of vegetation; therefore fires and especially wildfires
must be considered a minor source.
Aniline degrades in the atmosphere primarily by reaction
with photochemical-produced hydroxyl radicals, with a halflife
of 1–2 h. The reaction products include potentially harmful
substances such as nitrosamines, nitrobenzene, formic acid,
nitrophenols, phenol, nitrosobenzene, and benzidine. At
ground level, VOCs react with other air pollutants and
contribute to the formation of potentially harmful concentrations
of ozone in the lower atmosphere.
Aniline’s short half-life in air means that the likelihood of
long-range transport is low. | [Toxics Screening Level]
The Initial Threshold Screening Level (ITSL) for aniline is 1 μg/m3 (annual averaging
time). The Second ITSL is 76 μg/m3 (8 hour averaging time). The Initial Risk Screening
Level (IRSL) for aniline is 0.6 μg/m3 (annual averaging time). |
| Safety Data | Back Directory | [Hazard Codes ]
T,N,F | [Risk Statements ]
R23/24/25:Toxic by inhalation, in contact with skin and if swallowed .
R40:Limited evidence of a carcinogenic effect.
R41:Risk of serious damage to eyes.
R43:May cause sensitization by skin contact.
R48/23/24/25:Toxic: danger of serious damage to health by prolonged exposure through inhalation, in contact with skin and if swallowed .
R50:Very Toxic to aquatic organisms.
R68:Possible risk of irreversible effects.
R48/20/21/22:Harmful: danger of serious damage to health by prolonged exposure through inhalation, and in contact with skin and if swallowed .
R39/23/24/25:Toxic: danger of very serious irreversible effects through inhalation, in contact with skin and if swallowed .
R11:Highly Flammable. | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S27:Take off immediately all contaminated clothing .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S46:If swallowed, seek medical advice immediately and show this container or label .
S61:Avoid release to the environment. Refer to special instructions safety data sheet .
S63:In case of accident by inhalation, remove casualty to fresh air and keep at rest.
S36/37:Wear suitable protective clothing and gloves . | [OEL]
TWA: None ppm | [RIDADR ]
UN 1547 6.1/PG 2
| [WGK Germany ]
2
| [RTECS ]
BW6650000
| [F ]
8-9 | [Autoignition Temperature]
615 °C | [TSCA ]
Yes | [HazardClass ]
6.1 | [PackingGroup ]
II | [HS Code ]
29214110 | [storage]
Aniline should be kept stored against physical damage in a cool (but not freezing), dry,
well-ventilated location, away from smoking areas and fi re hazard. It should be kept separated from incompatibles and the containers should be bonded and grounded for transfer
to avoid static sparks | [Storage Class]
6.1A - Combustible acute toxic Cat. 1 and 2
very toxic hazardous materials | [Hazard Classifications]
Acute Tox. 3 Dermal
Acute Tox. 3 Inhalation
Acute Tox. 3 Oral
Aquatic Acute 1
Aquatic Chronic 1
Carc. 2
Eye Dam. 1
Muta. 2
Skin Sens. 1
STOT RE 1 | [Precautions]
When using aniline, occupational workers should wear impervious protective clothing,
including boots, gloves, laboratory coat, apron or coveralls, chemical safety goggles, and/
or a full face shield as appropriate, to prevent skin contact. Workplace facilities should
maintain an eye-wash fountain and quick-drench facilities. Workers should not eat, drink,
or smoke in the workplace. | [Safety Profile]
Suspected carcinogen
with experimental neoplastigenic data. A
human poison by an unspecified route.
Poison experimentally by most routes
incluhng inhalation and ingestion.
Experimental reproductive effects. A skin
and severe eye irritant, and a rmld sensitizer.
In the body, aniline causes formation of
methemoglobin, resulting in prolonged
anoxemia and depression of the central
nervous system; less acute exposure causes
hemolysis of the red blood cells, followed by
stimulation of the bone marrow. The liver
may be affected with resulting jaundice.
Long-term exposure to a d n e dye
manufacture has been associated with
malignant bladder growths. A common air
contaminant, A combustible liquid when
exposed to heat or flame. To fight fire, use
alcohol foam, CO2, dry chemical. It can
react vigorously with oxidizing materials.
When heated to decomposition it emits
highly toxic fumes of NOx. Spontaneously
explosive reactions occur with
benzenediazonium-2-carboxylate, dibenzoyl
peroxide, fluorine nitrate, nitrosyl
perchlorate, red fuming nitric acid,
peroxodisulfuric acid, and
tetranitromethane. Violent reactions with
boron trichloride, peroxyformic acid,
dhsopropyl peroxydicarbonate, fluorine,
trichloronitromethane (145℃), acetic
anhydride, chlorosulfonic acid,
hexachloromelamine, (HNO3 + N2O4 +
H2SO4), (nitrobenzene + glycerin), oleum,
(HCHO + HClO4), perchromates, K2O2, ppropiolactone,
AgClO4, Na2On, H2SO4,
trichloromelamine, acids, peroxydisulfuric
acid, F03Cl, diisopropyl peroxy-dicarbonate,
n-haloimides, and trichloronitromethane.
Ignites on contact with sodium peroxide +
water. Forms heator shock-sensitive
explosive mixtures with anhnium chloride (detonates at 240°C/7.6 bar), nitromethane,
hydrogen peroxide, 1 -chloro-2,3-
epoxypropane, and peroxomonosulfuric
acid. Reactions with perchloryl fluoride,
perchloric acid, and ozone form explosive
products. | [Hazardous Substances Data]
62-53-3(Hazardous Substances Data) | [Toxicity]
LD50 orally in rats: 0.44 g/kg (Jacobson) | [IDLA]
100 ppm |
| Raw materials And Preparation Products | Back Directory | [Raw materials]
Nitric acid-->Benzene-->Nitrogen-->Hydrogen-->Nitrobenzene-->Iron oxide | [Preparation Products]
8-Anilino-1-naphthalenesulfonic acid-->Direct Orange S-->Butyl 2-[[3-[[(2,3-dihydro-2-oxo-1H-benzimidazol-5-yl)amino]carbonyl]-2-hydroxy-1-naphthyl]azo]benzoate-->Poly(1,2-dihydro-2,2,4-trimethylquinoline)-->Pigment Red 175-->3-BROMOPYRIDINE-2-CARBOXYLIC ACID-->FLUORESCENT BRIGHTENER 28-->N,N-Bis(cyanoethyl)aniline-->4-N-DECYLANILINE-->4-bromo-2-(trifluoromethyl)quinoline-->Bronze Red-->Aniline hydrochloride-->Reactive Blue 222-->REACTIVE VIOLET 5-->sodium dibenzyl amine enzene sulfonate-->Dicyclohexylamine-->2-CHLOROMALONALDEHYDE-->N-PHENYLISONICOTINAMIDE-->Acid Yellow 79-->Acid Black 26-->UREA, N-(2,6-DIMETHYLPHENYL)-N'-[IMINO(METHYLAMINO)METHYL]--->2 BASIC ORANGE 2-->N-Phenyl-1-naphthylamine-->4-HYDROXY-2-(TRIFLUOROMETHYL)QUINOLINE-->SOLVENT BLACK 5-->Disperse Scarlet S-3GFL-->1,3-Diphenylurea-->N-(2-Naphthyl)aniline-->Acid Black 234-->LANASOLBLUE3R-->Direct Dark Brown NM-->Direct Bordeaux NGB-->Direct Green 89-->3-Hydroxydiphenylamine-->Phenylhydrazine sulfate-->2,4,6-Trichloroaniline-->2-Anilinoethanol-->Modified MDI-->Sudan I-->N,N-Diphenyl-p-phenylenediamine |
| Questions And Answer | Back Directory | [Description]
Aniline is the simplest primary aromatic amine and a compound formed by the substitution of a hydrogen atom in the benzene molecule with an amino group. It is colorless oil like flammable liquid with strong odor. When heated to 370 C, it is slightly soluble in water and soluble in ethanol, ether, chloroform and other organic solvents. It becomes brown in the air or under the sun. It can be distilled by steam. A small amount of zinc powder is added to prevent oxidation when it is distilled. The purified aniline can be added 10 ~ 15ppm NaBH4 to prevent oxidation deterioration. The solution of aniline is alkaline.
It is easy to produce salt when it reacts with acid. The hydrogen atoms on its amino groups can be substituted by alkyl or acyl groups to produce second or third grade aniline and acyl aniline. When substitution reaction occurs, the products of ortho and para substituted products are mainly produced. It reacts with nitrite to form diazonium salts, which can be used to produce a series of benzene derivatives and azo compounds.
 | [Uses]
Aniline is an important industrial chemical for many decades. Currently, it is most widely used for the manufacture of polyurethanes and rubber, with lesser amounts consumed in the production of pesticides (herbicides, fungicides, insecticides, animal repellants), defoliants, dyes, antioxidants, antidegradants, and vulcanization accelerators. It is also an ingredient of some household products, such as polishes (stove and shoe), paints, varnishes, and marking inks. | [Reaction]
A primary aromatic amine, aniline is a weak base and forms salts with mineral acids such as aniline hydrochloride. PKb = 9.30, 0.2mol aqueous solution PH value 8.1. In acidic solution, nitrous acid converts aniline into a diazonium salt that is an intermediate in the preparation of a great number of dyes and other organic compounds of commercial interest. When aniline is heated with organic acids, it gives amides, called anilides, such as acetanilide from aniline and acetic acid. Monomethylaniline and dimethylaniline can be prepared from aniline and methyl alcohol. Catalytic reduction of aniline yields cyclohexylamine.
Various oxidizing agents convert aniline to quinone, azobenzene, nitrosobenzene, p-aminophenol, and the phenazine dye aniline black. Amino groups can undergo acylation, halogenation, alkylation and diazotization, and the presence of amino groups makes it nucleophiles capable of many nucleophilic reactions, and at the same time activates the electrophilic substitution on aromatic rings.
| [Production]
Aniline was first obtained in 1826 by the destructive distillation of indigo. It is named because of the specific indigo-yielding plant “Indigofera anil” (Indigofera suffruticosa); In 1857, W.H.Jr. Perkin made aniline from reduction of nitrobenzene with iron filings using hydrochloric acid as catalyst which is still being used. At present, the methods of aniline production include catalytic vapor phase reduction of nitrobenzene with hydrogen, catalytic reaction of chlorobenzene and ammonolysis of phenol (Japan).
Before 1960s, aniline production was based on coal tar benzene, and now petroleum benzene has been used. At the end of 1990s, the world's aniline production capacity was above 2.5 million t. 50% of the aniline is used in the production of dye intermediates. About 25% aniline is used to produce isocyanate and its copolymers. The remaining (25%) is used for pesticides, gasoline antiknock agents, and photographic materials etc.
| [Hazards]
The toxicity of Aniline is LD50500mg/kg (dog oral administration), and is a common pollutant in the environment. Aniline has strong toxicity to blood and nerves. It can be absorbed by skin or by respiratory tract to cause toxicity.
The acute (short-term) and chronic (long-term) effects of aniline in humans consist mainly of effects on the lung, such as upper respiratory tract irritation and congestion. Chronic exposure may also result in effects on the blood. Human cancer data are insufficient to conclude that aniline is a cause of bladder tumors while animal studies indicate that aniline causes tumors of the spleen. EPA has classified aniline as a Group B2, probable human carcinogen.
Evidence reported by the National Institute for Occupational Safety and Health (NIOSH) clearly associates the occupational exposure to o-toluidine and aniline with an increased risk of bladder cancer among workers. The risk of bladder cancer is greatest among workers with possible and definite exposures to o-toluidine and aniline, and the risk increases with the duration of exposure.
|
|
|