1-Benzopyran-2-on Chemische Eigenschaften,Einsatz,Produktion Methoden
ERSCHEINUNGSBILD
FARBLOSE FLOCKEN MIT CHARAKTERISTISCHEM GERUCH.
ARBEITSPLATZGRENZWERTE
TLV nicht festgelegt.
MAK nicht festgelegt.
AUFNAHMEWEGE
Aufnahme in den Körper durch Inhalation des Aerosols, über die Haut und durch Verschlucken.
INHALATIONSGEFAHREN
Verdampfen bei 20°C vernachlässigbar; eine belästigende Partikelkonzentration in der Luft kann jedoch schnell erreicht werden.
WIRKUNGEN BEI KURZZEITEXPOSITION
WIRKUNGEN BEI KURZZEITEXPOSITION: Die Substanz reizt die Haut.
WIRKUNGEN NACH WIEDERHOLTER ODER LANGZEITEXPOSITION
Möglicherweise krebserzeugend für den Menschen.
Synthese
Als Reaktionsgefäß dient ein 100-ml-Dreihalskolben, der mit Rückflusskühler samt Calciumchlorid-Trockenrohr, Innenthermometer und Inertgaseinleitung (Argon) versehen wird. In dem Kolben werden 10 g (82 mmol) Salicylaldehyd, 16.3 ml (172 mmol) Essigsäureanhydrid und 6.7 g (82 mmol) zuvor entwässertes Natriumacetat vorgelegt. Mittels eines Ölbades wird der Kolbeninhalt unter Rückflusskühlung erhitzt. Während der achtstündigen Reaktionsdauer gewährleistet eine Ölbadtemperatur von ca. 180 ℃ die zur Reaktion notwendige Innentemperatur von ca. 150-160 ℃. Anschließend wird die noch heiße Reaktionsmasse mit dem gleichen Volumen Wasser versetzt und mit konzentrierter Salzsäure stark angesäuert. Die wässrige Phase wird dreimal mit je 40 ml Chloroform extrahiert. Die gesammelten organischen Phasen werden mit wasserfreiem Magnesiumsulfat getrocknet und das Trockenmittel über eine Fritte abgesaugt. Der so gewonnene Cumarin-Extrakt wird eingeengt, wobei rohes Cumarin anfällt.
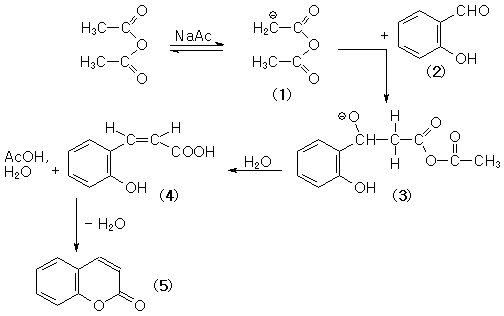
Zur Reaktion
Die aldolartige Umsetzung aromatischer Aldehyde mit Säureanhydriden wird als
Perkin-Reaktion bezeichnet. Im Allgemeinen wird die Reaktion durchgeführt, indem man den Aldehyd, das Säureanhydrid und das Natriumsalz der Carbonsäure mischt und mehrere Stunden auf 150-200 ℃ erhitzt.
LECKAGE
Reste sorgfältig sammeln. An sicheren Ort bringen. Persönliche Schutzausrüstung: Atemschutzgerät, P2-Filter für schädliche Partikel.
R-Sätze Betriebsanweisung:
R22:Gesundheitsschädlich beim Verschlucken.
R40:Verdacht auf krebserzeugende Wirkung.
R36/37/38:Reizt die Augen, die Atmungsorgane und die Haut.
R20/21/22:Gesundheitsschädlich beim Einatmen,Verschlucken und Berührung mit der Haut.
R43:Sensibilisierung durch Hautkontakt möglich.
S-Sätze Betriebsanweisung:
S36:DE: Bei der Arbeit geeignete Schutzkleidung tragen.
S36/37:Bei der Arbeit geeignete Schutzhandschuhe und Schutzkleidung tragen.
S26:Bei Berührung mit den Augen sofort gründlich mit Wasser abspülen und Arzt konsultieren.
Beschreibung
Coumarin is a naturally occurring Benzopyrone compound.
It is found in a large number of plants belonging to many
different families including tonka beans, woodruff, lavender
oil, cassia, melilot (sweet clover), and other plants. It is found
in edible plants such as strawberries, cinnamon, peppermint,
green tea, carrots, and celery, as well as in partially fermented
tea, red wine, beer, and other foodstuffs. Concentrations range
from 87 000 ppm in cassia and 40 000 ppm in cinnamon to
20 ppm in peppermint and 5 ppb in tangerines.
Chemische Eigenschaften
Coumarin occurs widely in nature and
determines, for example, the odor of woodruff. It forms white crystals (mp
70.6°C) with a hay-like, spicy odor. When treated with dilute alkali, coumarin is
hydrolyzed to the corresponding coumarinic acid salt [(Z)-2-hydroxycinnamic
acid]. Heating with concentrated alkali or with sodium ethanolate in ethanol
results in the formation of o-coumaric acid salts [(E)-2-hydroxycinnamic acid].
3,4-Dihydrocoumarin is obtained by catalytic hydrogenation, for example, with Raney nickel as a catalyst; octahydrocoumarin is obtained if hydrogenation is
carried out at high temperature (200–250°C).
Occurrence
Found in many plants and essential oils such as cassia, melilot, orchid, lavender and balsam of Peru (Sp?th, 1937; Gildemeister & Hoffman, 1966).
Verwenden
coumarin is considered a blood thinner, it can also increase blood flow. Some sources cite anti-oxidant capacities, as well. It is a specific plant constituent and is what creates the fragrance of freshly mowed hay. Coumarin is found in such plants as cherries, lavender, licorice, and sweet clover.
synthetische
Coumarin is currently produced by Perkin synthesis from salicylaldehyde.
In the presence of sodium acetate, salicylaldehyde reacts with acetic
anhydride to produce coumarin and acetic acid. The reaction is carried out in the
liquid phase at elevated temperature.
A process for the production of coumarin from hexahydrocoumarin by dehydrogenation has also been elaborated.
Since the odor of coumarin is relatively weak, strong-smelling by-products (e.g.,
vinylphenol) must be removed. Many purification methods have been reported
and patented.
Definition
A colorless crystalline compound
with a pleasant odor, used in making
perfumes. On hydrolysis with sodium
hydroxide it forms coumarinic acid.
Allgemeine Beschreibung
Colorless crystals, flakes or colorless to white powder with a pleasant fragrant vanilla odor and a bitter aromatic burning taste.
Air & Water Reaktionen
Insoluble in water.
Reaktivität anzeigen
Coumarin is sensitive to exposure to light. Coumarin is also sensitive to heat. Coumarin is incompatible with strong acids, strong bases and oxidizers. Coumarin is hydrolyzed by hot concentrated alkalis. Coumarin can be halogenated, nitrated and hydrogenated (in the presence of catalysts).
Hazard
Toxic by ingestion; carcinogenic. Use in
food products prohibited (FDA). Questionable carcinogen.
Health Hazard
SYMPTOMS: Exposure to Coumarin may cause narcosis. It may also cause irritation and liver damage.
Brandgefahr
Coumarin is combustible.
Biologische Aktivität
Oral anticoagulants can be prepared from compounds with coumarin as a base. Coumarin has been known for well over a century and, in addition to its use pharmaceutically, it is also an excellent odor-enhancing agent. However, because of its toxicity, it is not permitted in food products in the United States (Food and Drug Administration). One commercial drug is 3-(alpha-acetonyl-4-nitrobenzyl)- 4-hydroxycoumarin. This drug reduces the concentration of prothrombin in the blood and increases the prothrombin time by inhibiting the formation of prothrombin in the liver. The drug also interferes with the production of factors VII, IX, and X, so that their concentration in the blood is lowered during therapy. The inhibition of prothrombin involves interference with the action of vitamin K, and it has been postulated that the drug competes with vitamin K for an enzyme essential for prothrombin synthesis. Another commercial drug is bis-hydroxy-coumarin, C19H12O6. The actions of this drug are similar to those just described.
Kontakt-Allergie
Coumarin is an aromatic lactone naturally occurring in Tonka beans and other plants. As a fragrance allergen, it has to be mentioned by name in cosmetics within the EU
Sicherheitsprofil
Poison by ingestion,
intraperitoneal, and subcutaneous routes.
Questionable carcinogen with experimental
tumorigenic data. Experimental teratogenic
effects. Mutation data reported.
Combustible when exposed to heat or
flame. When heated to decomposition it
emits acrid smoke and fumes. See also
KETONES and ANHYDRIDES.
Environmental Fate
Coumarin toxicity is a function of blood and target tissue levels
of coumarin relative to the metabolic capacity of the target
organ. Cellular toxicity results when the formation of the toxic
moieties exceeds the capacity of the cell to detoxify. This can
have significant impact when comparing dosing by gavage to
dietary exposure.
läuterung methode
Coumarin crystallises from ethanol or water and sublimes in vacuo at 43o [Srinivasan & deLevie J Phys Chem 91 2904 1987]. [Beilstein 17/10 V 143.]
1-Benzopyran-2-on Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte

