Dinatriumcarbonat, Verbindung mitHydrogenperoxid (2:3) Chemische Eigenschaften,Einsatz,Produktion Methoden
R-Sätze Betriebsanweisung:
R8:Feuergefahr bei Berührung mit brennbaren Stoffen.
R22:Gesundheitsschädlich beim Verschlucken.
R41:Gefahr ernster Augenschäden.
R36/38:Reizt die Augen und die Haut.
S-Sätze Betriebsanweisung:
S17:Von brennbaren Stoffen fernhalten.
S26:Bei Berührung mit den Augen sofort gründlich mit Wasser abspülen und Arzt konsultieren.
S39:Schutzbrille/Gesichtsschutz tragen.
S37/39:Bei der Arbeit geeignete Schutzhandschuhe und Schutzbrille/Gesichtsschutz tragen.
Chemische Eigenschaften
Sodium percarbonate is a white granular powder of sodium carbonate and hydrogen peroxide.

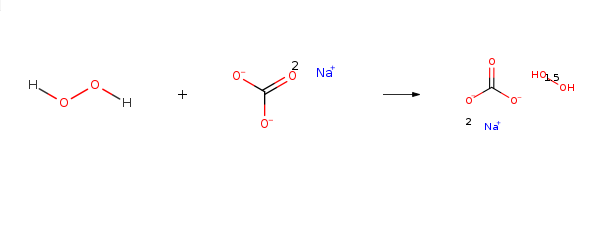
Sodium percarbonate is mainly used as a bleaching chemical in laundry detergents (tablets, compact or regular powders), laundry additives and machine dishwashing products. It is an oxidizing agent and ingredient in a number of home and laundry cleaning products, including eco-friendly bleach products such as OxiClean and Tide laundry detergent. Dissolved in water, it releases hydrogen peroxide and soda ash (sodium carbonate):
2(Na2CO31.5H202)→2Na2CO3+3H202
Verwenden
Sodium percarbonate is an addition salt of hydrogen peroxide and sodium carbonate that provides a solid source of hydrogen peroxide. When dissolved in water, sodium percarbonate liberates hydrogen peroxide. Sodium percarbonate is a white, granular or powdered solid oxidizer. It is used primarily as a bleaching agent in cleaning products. Other uses include algaecides, fungicides, chemical synthesis and environmental applications such as control of odor at waste treatment facilities. A small amount is used in denture cleaners and toothpaste.
Multifunctional reagent for the preparation of optically active 4-hydroxy-2-isoxazolines.
synthetische
Sodium percarbonate is produced by the reaction of sodium carbonate with hydrogen peroxide, which can be done via dry, spray and wet processes. In the dry process aqueous hydrogen peroxide solution is sprayed on solid sodium carbonate; a solid-liquid reaction yields sodium percarbonate. In the spray process sodium percarbonate is produced by a fluid bed process. Solutions of sodium carbonate and hydrogen peroxide are sprayed into a drying chamber where the water is evaporated. In the wet process sodium percarbonate is usually prepared by cristallisation possibly in combination with salting out.
Reaktionen
Sodium percarbonate naturally decomposes, very slowly, to form sodium carbonate and hydrogen peroxide. The hydrogen peroxide may further decompose to form water and oxygen and liberate some heat. The decomposition proceeds according to the reaction below:
2Na2CO3 • 3H2O2 → 2Na2CO3 + 3H2O + 1.5 O2 + Heat
Allgemeine Beschreibung
A colorless, crystalline solid. Denser than water. May combust in contact with organic materials. Contact may irritate skin, eyes and mucous membranes. May be toxic by ingestion. Used to make other chemicals.
Air & Water Reaktionen
Soluble in water.
Reaktivität anzeigen
Oxidizing agents, such as SODIUM PERCARBONATE, can react with reducing agents to generate heat and products that may be gaseous (causing pressurization of closed containers). The products may themselves be capable of further reactions (such as combustion in the air). The chemical reduction of materials in this group can be rapid or even explosive, but often requires initiation (heat, spark, catalyst, addition of a solvent). Explosive mixtures of inorganic oxidizing agents with reducing agents often persist unchanged for long periods if initiation is prevented. Such systems are typically mixtures of solids, but may involve any combination of physical states. Some inorganic oxidizing agents are salts of metals that are soluble in water; dissolution dilutes but does not nullify the oxidizing power of such materials. Organic compounds, in general, have some reducing power and can in principle react with compounds in this class. Actual reactivity varies greatly with the identity of the organic compound. Inorganic oxidizing agents can react violently with active metals, cyanides, esters, and thiocyanates.
Health Hazard
Inhalation, ingestion or contact (skin, eyes) with vapors or substance may cause severe injury, burns or death. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may cause pollution.
Brandgefahr
These substances will accelerate burning when involved in a fire. Some may decompose explosively when heated or involved in a fire. May explode from heat or contamination. Some will react explosively with hydrocarbons (fuels). May ignite combustibles (wood, paper, oil, clothing, etc.). Containers may explode when heated. Runoff may create fire or explosion hazard.
Sicherheitsprofil
Moderately toxic by ingestion. When heated to decomposition it emits acrid smoke and irritating vapors
Synthese
Sodium percarbonate is prepared by the reaction of dihydrogen peroxide and sodium carbonate. The specific synthesis steps are as follows:
With florisil In water addn. of small amounts of MgSiO3 and water glass to 500kg H2O2 (dild. to 20% soln.), addn. of 1051kg Na2CO3 at 18°C, cooling to 5°C, cooling to -4°C after addn. of 500kg NaCl; centrifugation, drying, reuse of mother-liquor, finally sodium percarbonate (yield: 85.5%) is obtained.
Environmental Fate
Sodium percarbonate is not persistent in the environment and readily decomposes to soda ash (sodium carbonate) and hydrogen peroxide which will subsequently decompose to water and oxygen when exposed to soils, sediments, and surface or ground waters.
Dinatriumcarbonat, Verbindung mitHydrogenperoxid (2:3) Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte

