Natriumhydroxid Chemische Eigenschaften,Einsatz,Produktion Methoden
ERSCHEINUNGSBILD
WEISSER, ZERFLIESSENDER FESTSTOFF IN VERSCHIEDENEN FORMEN, GERUCHLOS.
ARBEITSPLATZGRENZWERTE
TLV: (als STEL, ceiling) 2 mg/m?(ACGIH 2005). MAK: IIb (nicht festgelegt, aber Informationen vorhanden); (DFG 2005).
AUFNAHMEWEGE
Aufnahme in den Körper durch Inhalation des Aerosols und durch Verschlucken.
INHALATIONSGEFAHREN
Verdampfung bei 20°C vernachlässigbar; eine gesundheitsschädliche Partikelkonzentration in der Luft kann jedoch schnell erreicht werden.
WIRKUNGEN BEI KURZZEITEXPOSITION
WIRKUNGEN BEI KURZZEITEXPOSITION: ätzend. Die Substanz verätzt stark die Augen, die Haut und die Atemwege. ätzend beim Verschlucken. Inhalation des Aerosols der Substanz kann zu Lungenödem führen (s.Anm.).
WIRKUNGEN NACH WIEDERHOLTER ODER LANGZEITEXPOSITION
Wiederholter oder andauernder Hautkontakt kann Dermatitis hervorrufen.
LECKAGE
Verschüttetes Material in geeigneten Behältern sammeln; falls erforderlich durch Anfeuchten Staubentwicklung verhindern. Reste mit viel Wasser wegspülen. Persönliche Schutzausrüstung: Vollschutzanzug mit umgebungsluftunabhängigem Atemschutzgerät.
R-Sätze Betriebsanweisung:
R36/38:Reizt die Augen und die Haut.
R35:Verursacht schwere Verätzungen.
R34:Verursacht Verätzungen.
S-Sätze Betriebsanweisung:
S26:Bei Berührung mit den Augen sofort gründlich mit Wasser abspülen und Arzt konsultieren.
S45:Bei Unfall oder Unwohlsein sofort Arzt zuziehen (wenn möglich, dieses Etikett vorzeigen).
S37/39:Bei der Arbeit geeignete Schutzhandschuhe und Schutzbrille/Gesichtsschutz tragen.
S24/25:Berührung mit den Augen und der Haut vermeiden.
S36/37/39:Bei der Arbeit geeignete Schutzkleidung,Schutzhandschuhe und Schutzbrille/Gesichtsschutz tragen.
Aussehen Eigenschaften
Natronlauge 10,8 normal. Natriumhydroxidlösung 32 % ig.
Gefahren für Mensch und Umwelt
Mit Säuren erfolgt heftige, exotherme Reaktion, bei Kontakt mit Ammoniumsalzen wird Ammoniak freigesetzt! Beim Verdünnen mit Wasser erfolgt starke Erwärmung! NaOH verursacht schwere Verätzungen!
Starke lokale Ätzwirkung auf Haut, Augen und Schleimhäute. Gefahr der Hornhauttrübung mit Erblindungsgefahr. Perforation der Speiseröhre und des Magens möglich. Entstehung schlecht heilender Wunden.
Schutzmaßnahmen und Verhaltensregeln
Nebel und Stäube nicht einatmen! Jeden Kontakt mit der Haut vermeiden.
Schutzbrille mit Seitenschutz und oberer Augenraumabdeckung verwenden.
Latex- oder Neopren-Schutzhandschuhe (nur als kurzzeitiger Spritzschutz).
Verhalten im Gefahrfall
Verschüttete Substanz mit sehr viel Wasser wegspülen.
keine Einschränkung.
Stoff brennt selbst nicht; Kontakt mit Aluminium und Zink vermeiden (Explosionsgefahr durch entstehenden Wasserstoff).
Erste Hilfe
Nach Hautkontakt: Sofort mit viel Wasser abwaschen.
Nach Augenkontakt: 15 Minuten bei gespreizten Lidern unter fließendem Wasser mit Augendusche ausspülen. Augenarzt konsultieren!
Nach Einatmen: Frischluft. Arzt!
Nach Verschlucken: Wiederholt Wasser trinken. Erbrechen verhindern. Arzt!
Nach Kleidungskontakt: Verschmutzte bzw. benetzte Kleidung sofort ausziehen (ggf. auch Unterwäsche).
Ersthelfer: siehe gesonderten Anschlag
Sachgerechte Entsorgung
Nach vorsichtiger Neutralisation ins Abwasser geben.
Chemische Eigenschaften
Sodium hydroxide, NaOH,also referred to as caustic soda or sodium hydrate(and formerly known as lye), is a white,massive, deliquescent crystalline solid that is soluble in water,alcohol, and glycerol. It melts at 318°C (606 OF) and is the most widely used and available alkaline chemical. Most sodium hydroxide is produced as a coproduct of chlorine through the use of electrolytic cells;the cells are of the diaphragm, mercury, or membrane type. Some sodium hydroxide is marked as produced in the cells;most is evaporated and sold as 50% and 73% solutions or as anhydrous beads. Most caustic end uses require solutions of relatively low concentrations. Caustic soda is used as an analytical reagent and chemical intermediate, in scouring and cleaning baths,in rubber reclaiming and petroleum refining, in quenching baths for heat treating of steel,in cutting and soluble oils,in soaps and detergents, and in a wide variety of other applications.
Physikalische Eigenschaften
White orthorhombic crystals, produced in the form of pellets, lumps, sticks, beads, chips, flakes or solutions; hygroscopic; very corrosive; rapidly absorbs CO2 and water from the air; density 2.13 g/cm
3; melts at 323°C; vaporizes at 1388°C; vapor pressure 1 torr at 739°C and 5 torr at 843°C; very soluble in water (110 g/100mL at room temperature), generating heat on dissolution; aqueous solutions highly alkaline, pH of 0.5% solution about 13 and 0.05% solution about 12; soluble in methanol, ethanol and glycerol (23.8 g/100 mL methanol and 13.9 g/100 mL ethanol at ambient temperatures.).
Verwenden
Sodium hydroxide is one of the most important industrial chemicals. In volume, it is in the top ten chemicals produced in the United States. It is used in manufacturing a large number of compounds including several sodium salts, in treating cellulose for producing rayon and cellophane, and in manufacturing soaps, detergents, pulp, and paper. Sodium hydroxide is a common neutralizing agent for acids in acid-base titrations and petroleum refining. Another major application is extracting metals from their ores where alkali fusion, such as fusion with caustic soda, often is applied to open the ores. Additionally, sodium hydroxide is used to precipitate metals as hydroxides. Other uses are in reclaiming rubber, dissolving casein in plastics production, refining vegetable oils, processing textiles, as an eluant in ion chromatography, etching and electroplating, and as a laboratory reagent. Sodium hydroxide also is used as a strong base in many organic synthesis and base-catalyzed reactions.
Definition
The most important commercial
caustic.
Vorbereitung Methode
Sodium hydroxide is manufactured by electrolysis of brine using
inert electrodes. Chlorine is evolved as a gas at the anode and
hydrogen is evolved as a gas at the cathode. The removal of chloride
and hydrogen ions leaves sodium and hydroxide ions in solution.
The solution is dried to produce the solid sodium hydroxide.
A second method uses the Kellner–Solvay cell. Saturated sodium
chloride solution is electrolyzed between a carbon anode and a
flowing mercury cathode. In this case the sodium is produced at the
cathode rather than the hydrogen because of the readiness of
sodium to dissolve in the mercury. The sodium–mercury amalgam is
then exposed to water and a sodium hydroxide solution is
produced.
Reaktionen
Sodium hydroxide is strongly alkaline and can react with acids to form salts and water.
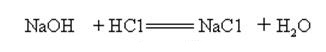
Sodium hydroxide reacts with acidic oxides to form salt and water, so sodium hydroxide can be used to absorb acid gases in the laboratory or industrially.
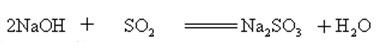
Sodium hydroxide can react with aqueous solutions of many metal salts to form sodium salts and metal hydroxides
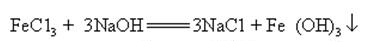
When sodium hydroxide and ammonia salt are heated together, it can release ammonia
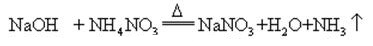
Sodium hydroxide is highly corrosive, so that the glass bottles storing sodium hydroxide solutions must be rubber stoppers, and glass stoppers should not be used to prevent a chemical reaction from opening. Sodium hydroxide is an important industrial raw material, and can be produced by electrolysis of saline solution industrially
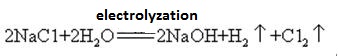
Allgemeine Beschreibung
A white solid. Corrosive to metals and tissue. Used in chemical manufacturing, petroleum refining, cleaning compounds, drain cleaners.
Air & Water Reaktionen
Soluble in water. Dissolution can liberate enough heat to cause steaming and spattering and ignite adjacent combustible material [Haz. Chem. Data 1966].
Hazard
Corrosive to tissue in presence of mois-
ture, strong irritant to tissue (eyes, skin, mucous
membranes, and upper respiratory tract), poison by
ingestion.
Health Hazard
Sodium hydroxide is a highly corrosive substancethat causes damage to human tissues.Its action on the skin is somewhat differentfrom acid burns. There is no immediate pain,but it penetrates the skin. It does not coagulateprotein to prevent its further penetration,and thus the caustic burn can become severeand slow healing. Spilling of its concentratedsolutions into the eyes can result in severeirritation or permanent injury.
It is toxic by ingestion as well as inhalationof its dust. Although the oral toxicity ofa 5–10% solution of caustic soda was foundto be low in test animals, high dosages atgreater concentrations can cause vomiting,prostration, and collapse. The oral lethal dosein rabbits is 500 mg/kg (NIOSH 1986).
Sodium hydroxide dusts or aerosols areirritating to the eyes, nose, and throat. Prolongedexposure to high concentrations in airmay produce ulceration of the nasal passage.
Brandgefahr
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Flammability and Explosibility
Sodium hydroxide and potassium hydroxide are not flammable as solids or aqueous
solutions.
Pharmazeutische Anwendungen
Sodium hydroxide is widely used in pharmaceutical formulations to
adjust the pH of solutions. It can also be used to react with weak
acids to form salts.
Industrielle Verwendung
Caustic soda (NaOH) is regarded as the strongest alkaline pH regulator. Caustic soda
is a very active substance and is highly corrosive. The bulk of caustic soda is manufactured
by electrolysis of saturated brines (NaCl). Caustic soda has a very strong pHregulating
capability (i.e. from pH 7 to pH 14) at a relatively low dosage compared to
other alkaline substances. Commercially, caustic soda is available in anhydrous form,
but in most mining applications the caustic soda is supplied as a 50% solution.
In the mineral processing industry, sodium hydroxide is mostly used for alkalinity control
during the processing of non-metallic minerals. In base metal flotation, the use of
sodium hydroxide is rare.
Sicherheitsprofil
Poison by intraperitoneal route. Moderately toxic by ingestion. Mutation data reported. A corrosive irritant to skin, eyes, and mucous membranes. When heated to decomposition it emits toxic fumes of NanO.
Sicherheit(Safety)
Sodium hydroxide is widely used in the pharmaceutical and food
industries and is generally regarded as a nontoxic material at low
concentrations. At high concentrations it is a corrosive irritant to
the skin, eyes, and mucous membranes.
LD50 (mouse, IP): 0.04 g/kg
LD50 (rabbit, oral): 0.5 g/kg
mögliche Exposition
NaOH is utilized to neutralize acids and make sodium salts in petroleum refining, viscose rayon; cellophane, plastic production; and in the reclamation of solutions of their salts. It is used in the manufacture of mercerized cotton, paper, explosives, and dyestuffs in metal cleaning; electrolytic extraction of zinc; tin plating; oxide coating; laundering, bleaching, dishwashing; and it is used in the chemical industries.
Lager
Sodium hydroxide should be stored in an airtight nonmetallic
container in a cool, dry place. When exposed to air, sodium
hydroxide rapidly absorbs moisture and liquefies, but subsequently
becomes solid again owing to absorption of carbon dioxide and
formation of sodium carbonate.
Versand/Shipping
UN1823 NaOH, solid, Hazard class: 8; Labels: 8-Corrosive material. UN1824 NaOH, solution, Hazard class: 8; Labels: 8-Corrosive material
Inkompatibilitäten
Sodium hydroxide is a strong base and is incompatible with any
compound that readily undergoes hydrolysis or oxidation. It will
react with acids, esters, and ethers, especially in aqueous solution.
Waste disposal
Discharge into tank containing water, neutralize, then flush to sewer with water.
Regulatory Status
GRAS listed. Accepted for use as a food additive in Europe.
Included in the FDA Inactive Ingredients Database (dental
preparations; injections; inhalations; nasal, ophthalmic, oral, otic,
rectal, topical, and vaginal preparations). Included in nonparenteral
and parenteral medicines licensed in the UK. Included in the
Canadian List of Acceptable Non-medicinal Ingredients.
Natriumhydroxid Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte
Sodium pyroantimonate
Disodium tartrate dihydrate
dibenzyl biphenyl polyoxyethylene ether
additive AC1210
2-(4,6-diamino-1,3,5-triazin-2-yl)acetic acid
2', 3'-ribonucleotide
3-(Acetylamino)thiophene-2-carboxylic acid
2-(1-Naphthalenyloxy)propanoic acid
3-Fluoro-4-hydroxybenzaldehyde
emulsifier C^{8~10^} OPE-10
thiourea-formaldehyde resin
Natrium-2-thenoat
11-Oxahexadecan-16-olid
4-METHYL-2-PHENYL-1,3-THIAZOLE-5-CARBOXYLIC ACID
Calcium dinatriumbis[2-chlor-5-[(2-hydroxy-1-naphthyl)azo]-4-sulfonatobenzoat]
DL-4-HYDROXY-3-METHOXYMANDELIC ACID
emulsifier SOPE-20
dodecyl phenyl polyoxyethylene (12) ether
5-Bromcytosin
C^{12~18^} fatty alcohol polyoxyethylene (35) ether
Alkaline Treated Starch
castor oil poloxyethylene (30) ether
5,5-Diethylbarbitursäure,Natrium-Salz
Tricobalttetraoxid
Natriumlactat
Sodium isoamylxanthate
Phosphorwolframsäure, Natrium-Salz
2-Hydroxy-1-naphthoesure
STRONTIUM HYDROXIDE OCTAHYDRATE
C^{8~9^} alkyl phenyl polyoxyethylene (18) ether
Chinuclidinhydrochlorid
Soda lime
4-Cyclohexen-1,3-dion, 6-β-D-Glucopyranosyl-2-[[3-β-D-glucopyranosyl-2,3,4-trihydroxy-5-[3-(4-hydroxyphenyl)-1-oxo-2-propenyl]-6-oxo-1,4-cyclohexadien-1-yl]methylen]-5,6-dihydroxy-4-[3-(4-hydroxyphenyl)-1-oxo-2-propenyl]-
SODIUM STANNATE TRIHYDRATE
2,3-Diphenylpropionsure
Natrium-O-isobutyldithiocarbonat
Peregal O-25
1-[(Benzyloxy)carbonyl]piperidine-4-carboxylic acid
2-AMINO-4,6-DIMETHOXY-1,3,5-TRIAZINE
BENZYL 1-PIPERAZINECARBOXYLATE

