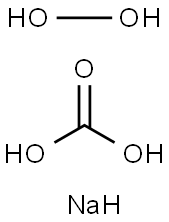
과탄산 나트륨
|
|
과탄산 나트륨 속성
- 밀도
- 2.09[at 20℃]
- 증기압
- < 10-3 Pa at 25°C
- 물리적 상태
- 과립분말
- 색상
- 하얀색
- 수소이온지수(pH)
- About 10.5 at 1% concentration (20°C)
- 냄새
- 냄새 없는
- 수용성
- 물에 용해됩니다.
- 감도
- Moisture Sensitive
- 분해온도
- > 131 °F (> 55 °C)
- LogP
- -0.809 (est)
- CAS 데이터베이스
- 15630-89-4(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | O,Xn | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 8-22-41-36/38 | ||
| 안전지침서 | 17-26-39-37/39 | ||
| 유엔번호(UN No.) | UN 1479 5.1/PG 3 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | FG0750000 | ||
| F 고인화성물질 | 21 | ||
| TSCA | Yes | ||
| 위험 등급 | 5.1 | ||
| 포장분류 | II | ||
| HS 번호 | 28369990 | ||
| 유해 물질 데이터 | 15630-89-4(Hazardous Substances Data) | ||
| 독성 | mouse,LD50,oral,2200mg/kg (2200mg/kg),BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY)LUNGS, THORAX, OR RESPIRATION: DYSPNEA,Toksikologicheskii Vestnik. Vol. (3), Pg. 46, 1994. | ||
| 기존화학 물질 | KE-05-0572 |
과탄산 나트륨 C화학적 특성, 용도, 생산
개요
과탄산소다는 소다회와 과산화수소를 원료로 사용하여 제조되는 백색 과립상의 제품으로 상온에서 안정한 알칼리성의 산소계 표백제입니다.용도
세제원료, 표백제, 세정제 원료, 폐수처리제, 산소발생제.화학적 성질
Sodium percarbonate is a white granular powder of sodium carbonate and hydrogen peroxide.
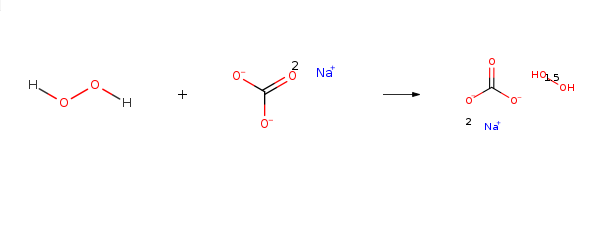
Sodium percarbonate is mainly used as a bleaching chemical in laundry detergents (tablets, compact or regular powders), laundry additives and machine dishwashing products. It is an oxidizing agent and ingredient in a number of home and laundry cleaning products, including eco-friendly bleach products such as OxiClean and Tide laundry detergent. Dissolved in water, it releases hydrogen peroxide and soda ash (sodium carbonate):
2(Na2CO31.5H202)→2Na2CO3+3H202
용도
Sodium percarbonate is an addition salt of hydrogen peroxide and sodium carbonate that provides a solid source of hydrogen peroxide. When dissolved in water, sodium percarbonate liberates hydrogen peroxide. Sodium percarbonate is a white, granular or powdered solid oxidizer. It is used primarily as a bleaching agent in cleaning products. Other uses include algaecides, fungicides, chemical synthesis and environmental applications such as control of odor at waste treatment facilities. A small amount is used in denture cleaners and toothpaste.Multifunctional reagent for the preparation of optically active 4-hydroxy-2-isoxazolines.
제조 방법
Sodium percarbonate is produced by the reaction of sodium carbonate with hydrogen peroxide, which can be done via dry, spray and wet processes. In the dry process aqueous hydrogen peroxide solution is sprayed on solid sodium carbonate; a solid-liquid reaction yields sodium percarbonate. In the spray process sodium percarbonate is produced by a fluid bed process. Solutions of sodium carbonate and hydrogen peroxide are sprayed into a drying chamber where the water is evaporated. In the wet process sodium percarbonate is usually prepared by cristallisation possibly in combination with salting out.화학 반응
Sodium percarbonate naturally decomposes, very slowly, to form sodium carbonate and hydrogen peroxide. The hydrogen peroxide may further decompose to form water and oxygen and liberate some heat. The decomposition proceeds according to the reaction below:2Na2CO3 • 3H2O2 → 2Na2CO3 + 3H2O + 1.5 O2 + Heat
일반 설명
A colorless, crystalline solid. Denser than water. May combust in contact with organic materials. Contact may irritate skin, eyes and mucous membranes. May be toxic by ingestion. Used to make other chemicals.공기와 물의 반응
Soluble in water.반응 프로필
Oxidizing agents, such as SODIUM PERCARBONATE, can react with reducing agents to generate heat and products that may be gaseous (causing pressurization of closed containers). The products may themselves be capable of further reactions (such as combustion in the air). The chemical reduction of materials in this group can be rapid or even explosive, but often requires initiation (heat, spark, catalyst, addition of a solvent). Explosive mixtures of inorganic oxidizing agents with reducing agents often persist unchanged for long periods if initiation is prevented. Such systems are typically mixtures of solids, but may involve any combination of physical states. Some inorganic oxidizing agents are salts of metals that are soluble in water; dissolution dilutes but does not nullify the oxidizing power of such materials. Organic compounds, in general, have some reducing power and can in principle react with compounds in this class. Actual reactivity varies greatly with the identity of the organic compound. Inorganic oxidizing agents can react violently with active metals, cyanides, esters, and thiocyanates.건강위험
Inhalation, ingestion or contact (skin, eyes) with vapors or substance may cause severe injury, burns or death. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may cause pollution.화재위험
These substances will accelerate burning when involved in a fire. Some may decompose explosively when heated or involved in a fire. May explode from heat or contamination. Some will react explosively with hydrocarbons (fuels). May ignite combustibles (wood, paper, oil, clothing, etc.). Containers may explode when heated. Runoff may create fire or explosion hazard.Safety Profile
Moderately toxic by ingestion. When heated to decomposition it emits acrid smoke and irritating vaporsSynthesis
Sodium percarbonate is prepared by the reaction of dihydrogen peroxide and sodium carbonate. The specific synthesis steps are as follows:With florisil In water addn. of small amounts of MgSiO3 and water glass to 500kg H2O2 (dild. to 20% soln.), addn. of 1051kg Na2CO3 at 18°C, cooling to 5°C, cooling to -4°C after addn. of 500kg NaCl; centrifugation, drying, reuse of mother-liquor, finally sodium percarbonate (yield: 85.5%) is obtained.
환경귀착
Sodium percarbonate is not persistent in the environment and readily decomposes to soda ash (sodium carbonate) and hydrogen peroxide which will subsequently decompose to water and oxygen when exposed to soils, sediments, and surface or ground waters.과탄산 나트륨 준비 용품 및 원자재
원자재
준비 용품
3-(6-FORMYL-PYRIDIN-2-YL)-BENZONITRILE
6-(3-NITROPHENYL)-2-PYRIDINECARBOXALDEHYDE
6-(4-METHYLSULFANYLPHENYL)PYRIDINE-2-CARBALDEHYDE
6-(4-METHOXYPHENYL)PYRIDINE-2-CARBALDEHYDE
6-(THIOPHEN-3-YL)PYRIDINE-2-CARBALDEHYDE
6-(4-FLUOROPHENYL)PYRIDINE-2-CARBALDEHYDE
4-(6-FORMYLPYRIDIN-2-YL)BENZONITRILE
6-(2-THIENYL)-2-PYRIDINECARBOXALDEHYDE
ETHYL 2-FLUOROPHENYLACETATE
6-(4-CHLOROPHENYL)PYRIDINE-2-CARBALDEHYDE
[Bis(trifluoroacetoxy)iodo]benzene
과탄산 나트륨 공급 업체
글로벌( 245)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hebei Jingbo New Material Technology Co., Ltd | +8619931165850 |
hbjbtech@163.com | China | 1000 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21691 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 |
info@tnjchem.com | China | 2989 | 55 |
| career henan chemical co | +86-0371-86658258 |
sales@coreychem.com | China | 29914 | 58 |
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 |
sales@sdzschem.com | China | 2931 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 |
sales@amoychem.com | China | 6387 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Shandong chuangyingchemical Co., Ltd. | 18853181302 |
sale@chuangyingchem.com | CHINA | 5909 | 58 |
| Shanghai Longyu Biotechnology Co., Ltd. | +8615821988213 |
info@longyupharma.com | China | 2531 | 58 |