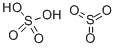FUMING SULFURIC ACID
- CAS No.
- 8014-95-7
- Chemical Name:
- FUMING SULFURIC ACID
- Synonyms
- H2SO4;SULPHURIC ACID;OLEUM;pyrosulfuric acid;OIL OF VITRIOL;SULFURIC ACID, FUMING;Sulphuric acid fuming;BETZ 0283;BETZ 0256;BETZ 0202
- CBNumber:
- CB0391001
- Molecular Formula:
- H2O7S2
- Molecular Weight:
- 178.14
- MDL Number:
- MFCD00137792
- MOL File:
- 8014-95-7.mol
| Melting point | 2 °C |
|---|---|
| Boiling point | ~290 °C(lit.) |
| Density | 1.925 g/mL at 25 °C(lit.) |
| vapor density | <0.3 (25 °C, vs air) |
| vapor pressure | 1 mm Hg ( 146 °C) |
| storage temp. | Store below +30°C. |
| solubility | H2O: soluble |
| form | Liquid |
| pka | 1.92(at 25℃) |
| color | Colorless |
| Specific Gravity | 1.925 |
| PH | <1 (100g/l, H2O, 20℃) |
| Water Solubility | Miscible with water. |
| Sensitive | Hygroscopic |
| Merck | 14,8974 |
| BRN | 2037554 |
| Exposure limits |
ACGIH: TWA 0.2 mg/m3 OSHA: TWA 1 mg/m3 NIOSH: IDLH 15 mg/m3; TWA 1 mg/m3 |
| Dielectric constant | 84(20.0℃) |
| Stability | Stable. Incompatible with organic materials, powdered metals, bases, halides. Reacts violently with water. Very hygroscopic. |
| CAS DataBase Reference | 8014-95-7(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | NTC1O8E83E |
| EPA Substance Registry System | Fuming sulfuric acid (8014-95-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS05,GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H314-H330-H335 | |||||||||
| Precautionary statements | P260-P271-P280-P303+P361+P353-P304+P340+P310-P305+P351+P338 | |||||||||
| Hazard Codes | C | |||||||||
| Risk Statements | 36/38-37-35-14-34 | |||||||||
| Safety Statements | 26-30-45-36/37/39 | |||||||||
| RIDADR | UN 3264 8/PG 3 | |||||||||
| OEB | C | |||||||||
| OEL | TWA: 1.0 mg/m3 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | WS5600000 | |||||||||
| F | 3 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 28070000 | |||||||||
| NFPA 704 |
|
FUMING SULFURIC ACID Chemical Properties,Uses,Production
Uses
Sulfuric acid probably is the most important industrial chemical of modern time. Most sulfuric acid manufactured is used by the fertilizer industry for making phosphoric acid and phosphate fertilizers.
Sulfuric acid has numerous applications. Some major uses include extracting ores; pickling metal; making explosives; manufacturing dyes, glues, and parchment papers; producing nitric and other acids; purifying petroleum; preparing metal sulfates; and synthesizing many organics. Sulfuric acid also is used in lead storage batteries for automobiles. The lead storage battery was invented by Gaston Plante in 1859. Sulfuric acid is used heavily in sulfonation, estertification, oxidation, dehydration, and acid-base neutralization reactions.
Sulfuric acid is a common laboratory reagent used for laboratory preparation of a large number of salts; as a dehydrating agent; as a component of chromic mixture for cleaning glassware; and in acid-base titration. The acid has been in wide usage in various industrial applications for more than two hundred years. Commercial concentrated acid has an assay of 95 to 98% H2SO4. Its normality is 36 N and density 1.834 to 1.836 g/mL.
Manufacture
Sulfuric acid is manufactured by two processes; namely, the chamber process and the contact process. The chamber process was discovered in 1746 and was used to produce sulfuric acid for over a century. This process was replaced by the contact process which has a lower production cost and yields a more concentrated acid needed for most industrial applications. The chamber process is obsolete now but for historical interest it is outlined below.
In the chamber process, nitric oxide catalyzes the oxidation of sulfur dioxide to trioxide:
The reaction is homogeneously catalyzed by NO. Although the oxidation process is exothermic and spontaneous, the reaction is very slow without a catalyst. The mechanism of the reaction is as follows:
2NO + O2 → 2NO2 (fast)
NO2 + SO2 → NO + SO3 (fast)
Practically all sulfuric acid is now made by the contact process. The starting material is sulfur dioxide, which is made by various methods, such as burning sulfur in dry air:
S (s) + O2( g) → SO2 (g)
or by burning pyrites or hydrogen sulfide:
4FeS2(s) + 11O2(g) → 8SO2 (g) + 2Fe2O3 (s)
2H2S (g) + 3O2 (g) → 2SO2 (g) + 2H2O(g)
Sulfur dioxide produced is reacted with oxygen in the presence of a catalyst to form sulfur trioxide:
2SO2 (g) + O2 (g) → 2SO3 (g)
Sulfur trioxide produced above by the contact process is absorbed in sulfuric acid to form pyrosulfuric acid, H2S2O7, which is diluted with water to form sulfuric acid:
SO3 (g) + H2SO4(l) → H2S2O7 (l)
H2S2O7 (l) + H2O (l) → 2H2SO4(l)
Sulfur trioxide also can be dissolved in water to form sulfuric acid. The dissolution of sulfur trioxide mist, however, is difficult to attain. Most plants employ sulfuric acid to dissolve SO3 vapor which can be diluted to obtain sulfuric acid of desired concentration.
Hazard
Sulfuric acid is a highly corrosive acid. Concentrated acid can cause severe burn on skin contact. Contact with eyes can damage vision.
Description
Fuming sulfuric acid is also called oleum, which is a trade name. It is a heavy, oily liquid, colorless to dark brown depending on purity, and fumes strongly in moist air and is extremely hygroscopic. Fuming sulfuric acid is a solution of sulfur trioxide in sulfuric acid. Sulfur trioxide is forced into solution with sulfuric acid to the point that the solution cannot hold any more. As soon as the solution is exposed to air, the fuming begins, forming dense vapor clouds. It is violently water reactive, as are most acids. Oleum is also a strong irritant to tissue. The four-digit UN identification number is 1831.
Chemical Properties
Sulfuric acid is a colorless to dark brown, odorless, oily liquid which is commercially sold @ 93% to 98% H2SO4, the remainder being water.
Chemical Properties
viscous liquid, is a mixture of sulfur trioxide dissolved in sulfuric acid. The SO3 content may range between 15 and 30%.
Physical properties
Dithionic acid, H2S2O6, is a chemical compound known only in solution. This acid is dibasic and salts called dithionates are known. All dithionates are readily soluble in water. They are mild oxidizing and mild reducing agents.
Uses
65% Sulfur Trioxide (Fuming) in Sulfuric Acid is a useful reagent that is widely used in the preparation of various compounds. It has been used for processing sulfonated polyoxadizole into ion-conducting films.
Uses
In manufacture of fertilizers, explosives, dyestuffs, other acids, parchment paper, glue, purification of petroleum, pickling of metal.
General Description
Thick fuming yellow liquid. Density 16.5 lb / gal. Very toxic by inhalation. Corrosive to metals and tissue, quickly causing severe burns. Used to make chemicals, dyes, explosives and in petroleum refining.
Air & Water Reactions
Fumes in air. Soluble in water; dissolution generates dangerous amounts of heat that can cause localized boiling and spattering of the acidic mixture and generate heavy fumes. During sulfonation of mononitrobenzene with fuming sulfuric acid, a leak from an internal cooling coil permitted water to enter the reaction tank. A violent eruption occurred due to the heat of solution [MCA Case History 944(1963)].
Reactivity Profile
SULFURIC ACID reacts as a strong acid, as an oxidizing agent and as a dehydrating agent. Chars wood, sugar and many other organic materials on contact. The heat from these reactions may ignite the wood, sugar or organic matter. May react explosively with acetic acid, acetic anhydride, acetonitrile, acrolein, acrylic acid, acrylonitrile, allyl alcohol, allyl chloride, ammonium hydroxide, aniline, cresol, butyraldehyde, cumene, ethyl acetate, ethylene diamine, ethylene glycol, glyoxal, isoprene, isopropyl alcohol, methyl ethyl ketone, propylene oxide, pyridine, styrene, vinyl acetate; strong bases (sodium hydroxide, potassium hydroxide) or mineral acids (nitric acid, hydrochloric acid, hydrofluoric acid) [Lewis, 3rd ed., 1993, p. 1195]. Mixing in equal molar portions with any of the following substances in a closed container caused the temperature and pressure to increase: 2-aminoethanol, ammonium hydroxide (28%), chlorosulfonic acid, ethylenediamine, ethyleneimine, ethylene cyanohydrin, hydrochloric acid (36%), hydrofluoric acid (48.7%), isopropyl alcohol, nitric acid (70%), 2-nitropropane, propiolactone, propylene oxide, pyridine, styrene monomer, sodium hydroxide, sulfolane, vinyl acetate, vinylidene chloride [NFPA 1991]. Extremely hazardous in contact with carbides, bromates, chlorates, fulminates, picrates, and powdered metals. May induce violent polymerization in polymerizable organic compounds such as allyl chloride. Reacts exothermically with sodium hypochlorite to produce chlorine gas.
Hazard
Reacts violently with water. Strong irritant to tissue.
Health Hazard
Acid mist is irritating to eyes, nose and throat. Liquid causes severe burns of skin and eyes.
Health Hazard
Oleum is an extremely corrosive substance. It emits suffocating fumes of sulfur trioxide that can cause severe lung damage. Skin contact can cause severe burns.
LC50 value, inhalation (rats): 347 ppm/L/h.
Fire Hazard
Special Hazards of Combustion Products: Toxic and irritating vapors are generated.
Safety Profile
Confirmed human carcinogen. A poison. Moderately toxic by inhalation. A corrosive irritant to skin, eyes, and mucous membranes. A very dangerous fire hazard by chemical reaction with reducing agents and carbohydrates. A severe explosion hazard by chemical reaction with acetic acid, acetic anhydride, acetonitrile, acrolein, acrylic acid, acrylonitrile, allylalcohol, allyl chloride, 2-amino ethanol, NH4OH, aniline, cresol, n-butyraldehyde, cumene, dichloroethyl ether, diethylene glycol monomethyl ether, diisobutylene, epichlorohydrin, ethyl acetate, ethylene cyanohydrin, ethylene diamine, ethylene glycol, ethylene glycol monoethyl ether acetate, ethylene imine, glyoxal, HCl, HF, isoprene, isopropyl alcohol, mesityl oxide, methyl ethyl ketone, HNO3, 2-nitropropane7 p-propiolacetone, propylene oxide, pyridine, NaOH, styrene monomer, vinylidene chloride, sulfolane, vinyl acetate. Will react with water or steam to produce heat and toxic and corrosive fumes. Can react vigorously with reducing materials. When heated to decomposition it emits highly toxic fumes of SOx. See also SULFUROUS ACID.
Potential Exposure
Used as a chemical feedstock in the manufacture of acetic acid, hydrochloric acid; citric acid; phosphoric acid; aluminum sulfate; ammonium sulfate;barium sulfate; copper sulfate; phenol, superphosphates, titanium dioxide; as well as synthetic fertilizers, nitrate explosives; artificial fibers; dyes, pharmaceuticals, detergents, glue, paint, and paper. It finds use as a dehydrating agent for esters and ethers due to its high affinity for water; as an electrolyte in storage batteries; for the hydrolysis of cellulose to obtain glucose; in the refining of mineral and vegetable oil; and in the leather industry. Other uses include fur and food processing; carbonization of wool fabrics; gas drying; uranium extraction from pitchblende; and laboratory analysis. Sulfuric acid is among the highestvolume produced chemical in the United States.
Shipping
UN1830 Sulfuric acid with >51% acid or sulfuric acid with not >51% acid, Hazard class: 8; Labels: 8-Corrosive material. UN1831 Sulfuric acid, fuming with 30% or more free sulfur trioxide and Sulfuric acid, fuming, with <30% free sulfur trioxide, Hazard class: 8; Labels: 8-Corrosive material. UN1832 Sulfuric acid, spent, Hazard class: 8; Labels: 8-Corrosive material.
Incompatibilities
A strong acid and oxidizer. Reacts violently with water with dangerous spattering and evolution of heat. Reacts violently with combustible and reducing materials; bases, organic materials; chlorates, carbides, picrates, fulminates, water, powdered metals. Corrosive to most common metals forming explosive hydrogen gas.
Waste Disposal
Add slowly to solution of soda ash and slaked lime with stirring; flush to drain with large volumes of water. Recovery and reuse of spent sulfuric acid may be a viable alternative to disposal, and processes are available.
FUMING SULFURIC ACID Preparation Products And Raw materials
Raw materials
Preparation Products
1of8