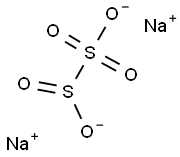
Sodium metabisulfite
- CAS No.
- 7681-57-4
- Chemical Name:
- Sodium metabisulfite
- Synonyms
- Sodium pyrosulfite;Metabisulfite Sodium;Sodium pyrosulphite;Sodium disulphite;E223;Sodiummetabisufite;Disodium disulphite;fertisilo;Sodium metabisuL;SodiuM pyrosulfit
- CBNumber:
- CB9854280
- Molecular Formula:
- H2O5S2.2Na
- Molecular Weight:
- 192.12
- MDL Number:
- MFCD00213787
- MOL File:
- 7681-57-4.mol
- MSDS File:
- SDS
| Melting point | >300 °C (lit.) |
|---|---|
| Density | 1.48 |
| storage temp. | Store at +5°C to +30°C. |
| solubility | 540 g/L (20°C) |
| form | Powder/Solid |
| Specific Gravity | 1.48 |
| color | White to slightly yellow |
| Odor | Sulphorous |
| PH Range | 4.5 at 50 g/l at 20 °C |
| PH | 3.5-5 (50g/l, H2O, 20℃) |
| Water Solubility | 540 g/L (20 ºC) |
| Sensitive | Air & Moisture Sensitive |
| Merck | 14,8638 |
| Exposure limits |
ACGIH: TWA 5 mg/m3 NIOSH: TWA 5 mg/m3 |
| Stability | Stable. Incompatible with strong oxidizing agents, strong acids. Contact with strong acids releases a poisonous gas. May be moisture and air sensitive. |
| InChIKey | HRZFUMHJMZEROT-UHFFFAOYSA-L |
| CAS DataBase Reference | 7681-57-4(CAS DataBase Reference) |
| FDA 21 CFR | 182.3766; 582.3766; 173.310 |
| Substances Added to Food (formerly EAFUS) | SODIUM METABISULFITE |
| SCOGS (Select Committee on GRAS Substances) | Sodium metabisulfite |
| EWG's Food Scores | 2-4 |
| FDA UNII | 4VON5FNS3C |
| EPA Substance Registry System | Sodium metabisulfite (7681-57-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS05,GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H302-H318 | |||||||||
| Precautionary statements | P264-P270-P280-P301+P312-P305+P351+P338-P501 | |||||||||
| Hazard Codes | Xn,Xi | |||||||||
| Risk Statements | 22-31-41-36/37/38-52 | |||||||||
| Safety Statements | 26-39-46-36-16 | |||||||||
| RIDADR | 3260 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | UX8225000 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 2832 10 00 | |||||||||
| PackingGroup | III | |||||||||
| Toxicity | LD50 orally in Rabbit: 1540 mg/kg LD50 dermal Rat > 2000 mg/kg | |||||||||
| NFPA 704 |
|
Sodium metabisulfite price More Price(48)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 13459 | Sodium metabisulfite puriss., meets analytical specification of Ph. Eur., BP, NF, FCC, E223, dry, 97-100.5% | 7681-57-4 | 500g | $46.6 | 2024-03-01 | Buy |
| Sigma-Aldrich | 13459 | Sodium metabisulfite puriss., meets analytical specification of Ph. Eur., BP, NF, FCC, E223, dry, 97-100.5% | 7681-57-4 | 1kg | $57.1 | 2024-03-01 | Buy |
| Sigma-Aldrich | 13459 | Sodium metabisulfite puriss., meets analytical specification of Ph. Eur., BP, NF, FCC, E223, dry, 97-100.5% | 7681-57-4 | 5kg | $133.8 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1.06528 | Sodium disulfite (sodiummetabisulfite)foranalysisEMSURE?ACS,Reag.PhEur | 7681-57-4 | 100g | $43.3 | 2024-03-01 | Buy |
| Sigma-Aldrich | 13459 | Sodium metabisulfite puriss., meets analytical specification of Ph. Eur., BP, NF, FCC, E223, dry, 97-100.5% | 7681-57-4 | 4x5kg | $724 | 2024-03-01 | Buy |
Sodium metabisulfite Chemical Properties,Uses,Production
Description
Sodium metabisulfite (chemical formula: Na2S2O5) appears as a white crystalline or powder solid with a slight sulfur odor. It is toxic upon inhalation and can strongly irritate skin and tissue. It can be decomposed to release toxic oxide fumes of sulfur and sodium upon high temperature. It can be mixed with water to form a corrosive acid. It is generally used as disinfectant, antioxidant, and preservative agent as well as a laboratory reagent. As a kind of food additive, it can be used as a preservative and antioxidant in food. It can also be applied to the wine and beer making. Moreover, it can be used to sanitize the equipment of homebrew and winemaking as a cleaning agent. It also have various kinds of other applications, e.g. being applied to photography, as an excipients in some tablets, for water treatment, as a source of SO2 in wine, as a bactericide and as a bleaching reagent as well as reducing agent. It can be manufactured through the evaporation of a sodium bisulfite which has been saturated with sulfur dioxide. It should be warned that sodium metabisulfite has certain acute effects on the respiratory system, eyes and skin. In severe case, it can cause breathing difficulty and even pulmonary damage which finally leads to death. Therefore, effective protective measures and attention should be taken during the operation.

sodium metabisulfite powder
Uses
Sodium metabisulfite (SMBS, Sodium disulfite) is a white, granular solid sodium salt. an inorganic compound made up of sodium, sulfur, and oxygen, and used in many industries:
- in the pulp and paper industry, in the photographic industry and in the various other industries as a bleach or dechlorinator.
- Food Grade sodium metabisulfite may be used as a food preservative. It is also commonly added to various food products and wines as a preservative.
- Sodium metabisulfite can also be used in the manufacture of other chemicals, Used in production of cleaning agents, detergents, and soaps.
- It also acts as a corrosion inhibitor in oil and gas industry, as a bleaching agent in the production of coconut cream, as the source of sulfur dioxide and in the destruction of cyanide in commercial gold cyanidation processes.
- Gold mining industry: It is utilized in the precipitation of gold from auric acid as well as in the waste water treatment to remove hexavaent chromium as trivalent chromium by precipitation after reduction.
- Preservative in photo developer solutions, it is used in photography.
- Oxygen scavenge:it plays as an oxygen scavenger to eliminate the dissolved oxygen in wastewater and in pipes.
- Sodium metabisulfite may be used as an initiator during the cross-linking polymerization of polybutadiene in the cores of the vesicle membranes.
- It may be added as an antioxidant during the preparation of stock solutions of 6-hydroxydopamine in various studies.
- Dechlorination in municipal wastewater, pulp & paper, power, and textile water treatment plants.
Preparation
manufactures sodium metabisulfite by reacting sulfur dioxide with sodium carbonate (soda ash), purifying and drying to form crystals or powder.
Na2CO3 + 2SO2→Na2S2O5 + CO2
Product Specification
Health Hazard
Sodium metabisulphite is currently listed in Annex III Part B of Directive 95/2/EC as an authorised food additive. As such food labelling is required where sulphite residues exceed 10 mg/kg (ppm). This is labelled E223 with maximum permitted residues in crustacean products set at 150 mg/kg. However it is now accepted that sulphites, when present in foodstuffs, can cause allergic reactions in vulnerable persons and can constitute a danger to health (Collins-Williams, 1983). Metabisulphite is regarded as one causative agent of asthma attacks (Gunnison & Jacobsen, 1987). In accordance with this, EU Directive 2003/89/EC will make allergen labelling a requirement for all food stuffs produced in the European Community, which have been treated with sodium metabisulphite.
Sodium metabisulfite typically found in consumer products should pose little a risk of symptoms due to skin or inhalation exposure since sodium metabisulfite is used in very low concentrations. Sodium metabisulfite can produce the following adverse health affects:
- Contact - Skin exposures can cause symptoms ranging from minor skin irritation or itching to redness and swelling. Eye exposure to sodium metabisulfite may result in redness, tearing or moderate eye irritation.
- Inhalation - The inhalation of sodium metabisulfite dusts can cause nose and throat irritation or coughing. Repeated or prolonged exposures may cause sore throat or nosebleeds. Inhalation may also cause severe respiratory reactions and aggravate asthma or other breathing diseases.
- Ingestion - The ingestion of sodium metabisulfite may cause irritation of the mouth and throat, nausea, vomiting and diarrhea.
- Other Effects - The International Agency for Research on Cancer (IARC) has not classified sodium metabisulfite as a carcinogen (cancer causing).
References
http://msdssearch.dow.com/PublishedLiteratureDOWCOM/dh_004f/0901b8038004f697.pdf
https://en.wikipedia.org/wiki/Sodium_metabisulfite
https://pubchem.ncbi.nlm.nih.gov/compound/Sodium_metabisulfite#section=Metabolism-Metabolites
Chemical Properties
Sodium metabisulfite occurs as colorless, prismatic crystals or as a white to creamy-white crystalline powder that has the odor of sulfur dioxide and an acidic, saline taste. Sodium metabisulfite crystallizes from cold water as a hydrate containing seven water molecules.
Chemical Properties
Sodium metabisulfite is a white crystalline powder. Sulfur dioxide odor. It may be considered the anhydride of 2 molecules of sodium disulfite.
Uses
Sodium Metabisulfite is a preservative and antioxidant that exists as crystals or powder having a sulfur dioxide odor. it is readily solu- ble in water. it is used in dried fruits to preserve flavor, color, and to inhibit undesirable microorganism growth. it prevents “black spots” due to oxidative deterioration in shrimp. it is used in maraschino cherries. it is found in lemon drinks as a preservative. see sulfur dioxide.
Uses
Pharmaceutic aid (antioxidant).
Uses
sodium metabisulfite is an anti-oxidant and reducing agent.
Definition
ChEBI: An inorganic sodium salt composed of sodium and disulfite ions in a 2:1 ratio.
Production Methods
Sodium metabisulfite is prepared by saturating a solution of sodium hydroxide with sulfur dioxide and allowing crystallization to occur; hydrogen is passed through the solution to exclude air. Sodium metabisulfite may also be prepared by saturating a solution of sodium carbonate with sulfur dioxide and allowing crystallization to occur, or by thermally dehydrating sodium bisulfite.
General Description
Sodium metabisulfite (MBS, Sodium disulfite) is an inorganic salt. It is the sodium salt of disulphurous (pyrosulfurous) acid. It is widely used in textile dyeing, photography and paper industry. It is also commonly added to various food products and wines as a preservative.
Hazard
Toxic by inhalation. Upper respiratory tract irritant. Questionable carcinogen.
Health Hazard
Sodium metabisulfite may cause bronchospasm, oculonasal symptoms, and urticaria in sulfite-sensitive individuals; irritation of mucous membranes may occur from inhalation of the dust.
Flammability and Explosibility
Non flammable
Pharmaceutical Applications
Sodium metabisulfite is used as an antioxidant in oral, parenteral,
and topical pharmaceutical formulations, at concentrations of
0.01–1.0% w/v, and at a concentration of approximately 27% w/v
in intramuscular injection preparations. Primarily, sodium metabisulfite
is used in acidic preparations; for alkaline preparations,
sodium sulfite is usually preferred. Sodium
metabisulfite also has some antimicrobial activity, which is greatest
at acid pH, and may be used as a preservative in oral preparations
such as syrups.
In the food industry and in wine production, sodium metabisulfite
is similarly used as an antioxidant, antimicrobial preservative,
and antibrowning agent. However, at concentrations above
about 550 ppm it imparts a noticeable flavor to preparations.
Sodium metabisulfite usually contains small amounts of sodium
sulfite and sodium sulfate.
Contact allergens
This agent is frequently used as a preservative in pharmaceutical products, in the bread-making industry as an antioxidant, and it can induce contact dermatitis. It can be used as a reducing agent in photography and caused dermatitis in a photographic technician, probably acting as an aggravating irritative factor. Sodium metabisulfite contains a certain amount of sodium sulfite and sodium sulfate.
Safety Profile
An inhalation hazard. Poison by intravenous route. Moderately toxic by parenteral route. Experimental reproductive effects. Mutation data reported. When heated to decomposition it emits toxic fumes of SOx and Na2O.
Safety
Sodium metabisulfite is widely used as an antioxidant in oral,
topical, and parenteral pharmaceutical formulations; it is also
widely used in food products.
Although it is extensively used in a variety of preparations,
sodium metabisulfite and other sulfites have been associated with a
number of severe to fatal adverse reactions. These are usually
hypersensitivity-type reactions and include bronchospasm and
anaphylaxis. Allergy to sulfite antioxidants is estimated to occur
in 5–10% of asthmatics, although adverse reactions may also occur
in nonasthmatics with no history of allergy.
Following oral ingestion, sodium metabisulfite is oxidized to
sulfate and is excreted in urine. Ingestion may result in gastric
irritation, owing to the liberation of sulfurous acid, while ingestion
of large amounts of sodium metabisulfite can cause colic, diarrhea,
circulatory disturbances, CNS depression, and death.
In Europe, the acceptable daily intake of sodium metabisulfite
and other sulfites used in foodstuffs has been set at up to 3.5 mg/kg
body-weight, calculated as sulfur dioxide (SO2). The WHO has
similarly also set an acceptable daily intake of sodium metabisulfite,
and other sulfites, at up to 7.0 mg/kg body-weight, calculated as
sulfur dioxide (SO2).
LD50 (rat, IV): 0.12 g/kg
Potential Exposure
Sodium metabisulfite is used as an antioxidant in pharmaceutical preparations and as a preservative in foods. People with asthma have a greater chance of having an allergic reaction with this chemical. Individuals allergic to sodium bisulfite (a food preservative found in some wine, fresh shrimp; packaged foods; and restaurant salads and potatoes) may have a severe reaction when exposed to sodium metabisulfite.
Carcinogenicity
Sodium metabisulfite was genotoxic in mice in vivo as determined by chromosomal aberration, micronucleus, and sperm shape assays. It was not mutagenic in bacterial assays.
storage
On exposure to air and moisture, sodium metabisulfite is slowly
oxidized to sodium sulfate with disintegration of the crystals.
Addition of strong acids to the solid liberates sulfur dioxide.
In water, sodium metabisulfite is immediately converted to
sodium (Na+) and bisulfite (HSO3-) ions. Aqueous sodium
metabisulfite solutions also decompose in air, especially on heating.
Solutions that are to be sterilized by autoclaving should be filled into
containers in which the air has been replaced with an inert gas, such
as nitrogen. The addition of dextrose to aqueous sodium
metabisulfite solutions results in a decrease in the stability of the
metabisulfite.
The bulk material should be stored in a well-closed container,
protected from light, in a cool, dry place.
Shipping
UN1759 Corrosive solids, n.o.s., Hazard class: 8; Labels: 8-Corrosive material, Technical Name required. UN2693 Bisulfites, inorganic, aqueous solutions, n.o.s., Hazard class: 8; Labels: 8-Corrosive material.
Incompatibilities
Sodium metabisulfite reacts with sympathomimetics and other
drugs that are ortho- or para-hydroxybenzyl alcohol derivatives to
form sulfonic acid derivatives possessing little or no pharmacological
activity. The most important drugs subject to this inactivation
are epinephrine (adrenaline) and its derivatives. In addition,
sodium metabisulfite is incompatible with chloramphenicol owing
to a more complex reaction; it also inactivates cisplatin in
solution.
It is incompatible with phenylmercuric acetate when autoclaved
in eye drop preparations.
Sodium metabisulfite may react with the rubber caps of
multidose vials, which should therefore be pretreated with sodium
metabisulfite solution.
Incompatibilities
A strong reducing agent. Keep away from oxidizers. Mixtures with water forms a strong corrosive. Contact with acids releases toxic fumes. Heat causes decomposition. Slowly oxidized to the sulfate on exposure to air and moisture. Attacks metals
Regulatory Status
GRAS listed. Accepted for use as a food additive in Europe. Included in the FDA Inactive Ingredients Database (epidural;inhalation; IM and IV injections; ophthalmic solutions; oral preparations; rectal, topical, and vaginal preparations). Included in nonparenteral and parenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.
Sodium metabisulfite Preparation Products And Raw materials
Raw materials
Preparation Products
1of8
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418684 +8618949823763 | sales@tnjchem.com | China | 25363 | 58 |
| Hebei Dongdu Import and Export Co. LTD | +86-15333296769 +86-15333296769 | manager@cndongdu.com | China | 71 | 58 |
| Hebei Yime New Material Technology Co., Ltd. | +86-66697723 +86-17703311139 | admin@china-yime.com | China | 563 | 58 |
| Hebei Dangtong Import and export Co LTD | +8615632927689 | admin@hbdangtong.com | China | 991 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | deasea125996@gmail.com | China | 2503 | 58 |
| Shandong Juchuang Chemical Co., LTD | +86-18885615001 +86-18885615001 | admin@juchuangchem.com | China | 387 | 58 |
| Hebei Kangcang new material Technology Co., LTD | +8619133911216 | fanfan@kangcang.com.cn | China | 340 | 58 |
| Hebei Saisier Technology Co., LTD | +86-18400010335 +86-13102810335 | admin@hbsaisier.cn | China | 818 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 15933 | 58 |
Related articles
- Sodium Metabisulfite: Uses, Synthesis and Hazards
- The passage introduces the uses, synthesis and hazards of sodium metabisulfite, also mention the reaction with water of it.
- Nov 24,2022
- a challenging allergen:sodium metabisulfite
- Sodium metabisulfite appears as a white crystalline or powder solid with a slight sulfur odor. Toxic by inhalation. Sodium dis....
- Apr 11,2022
View Lastest Price from Sodium metabisulfite manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-28 | Sodium metabisulfite
7681-57-4
|
US $500.00 / T | 100T | 99% | 20tons/day | Hebei Yime New Material Technology Co., Ltd. | |
 |
2024-04-28 | Sodium metabisulfite
7681-57-4
|
US $9.00-70.00 / g | 10g | 99% | 10 tons | Hebei Kangcang new material Technology Co., LTD | |
 |
2024-04-28 | Sodium metabisulfite
7681-57-4
|
US $230.00 / ton | 5ton | 97% | 3000tons | Hebei Dangtong Import and export Co LTD |
-

- Sodium metabisulfite
7681-57-4
- US $500.00 / T
- 99%
- Hebei Yime New Material Technology Co., Ltd.
-

- Sodium metabisulfite
7681-57-4
- US $9.00-70.00 / g
- 99%
- Hebei Kangcang new material Technology Co., LTD
-

- Sodium metabisulfite
7681-57-4
- US $230.00 / ton
- 97%
- Hebei Dangtong Import and export Co LTD