(+)-Pilocarpine hydrochloride
- CAS No.
- 54-71-7
- Chemical Name:
- (+)-Pilocarpine hydrochloride
- Synonyms
- PILOCARPINE HCL;PILOCARPINE HYDROCHLORIDE;(3S,4R)-4,5-DIHYDRO-3-ETHYL-4-(1-METHYL-1H-IMIDAZOL-5-YLMETHYL)-2(3H)-FURANONE HYDROCHLORIDE;Salagen;Sanpilo;pilocar;pilocel;(3S,4R)-;Akarpine;ami-pilo
- CBNumber:
- CB1236112
- Molecular Formula:
- C11H17ClN2O2
- Molecular Weight:
- 244.72
- MDL Number:
- MFCD00012722
- MOL File:
- 54-71-7.mol
| Melting point | 202-205 °C(lit.) |
|---|---|
| alpha | 89 º (C=5, H2O) |
| storage temp. | 2-8°C |
| solubility | H2O: 100 mg/mL |
| form | powder |
| color | white |
| Water Solubility | soluble |
| Sensitive | Hygroscopic |
| Merck | 14,7424 |
| BRN | 4034491 |
| Stability | Hygroscopic |
| LogP | -0.095 (est) |
| CAS DataBase Reference | 54-71-7(CAS DataBase Reference) |
| FDA UNII | 0WW6D218XJ |
| NCI Drug Dictionary | pilocarpine hydrochloride |
| EPA Substance Registry System | Pilocarpine monohydrochloride (54-71-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H300+H330 | |||||||||
| Precautionary statements | P301+P310+P330-P304+P340+P310 | |||||||||
| Hazard Codes | T+,T | |||||||||
| Risk Statements | 26/28-25-23 | |||||||||
| Safety Statements | 25-45 | |||||||||
| RIDADR | UN 1544 6.1/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | TK1450000 | |||||||||
| F | 3-8-10 | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29399990 | |||||||||
| NFPA 704 |
|
(+)-Pilocarpine hydrochloride price More Price(27)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 1538902 | Pilocarpine hydrochloride United States Pharmacopeia (USP) Reference Standard | 54-71-7 | 200mg | $436 | 2024-03-01 | Buy |
| Sigma-Aldrich | P0472 | Pilocarpine hydrochloride meets USP testing specifications | 54-71-7 | 10g | $423 | 2022-05-15 | Buy |
| Cayman Chemical | 14487 | (+)-Pilocarpine (hydrochloride) ≥98% | 54-71-7 | 1g | $48 | 2024-03-01 | Buy |
| Cayman Chemical | 14487 | (+)-Pilocarpine (hydrochloride) ≥98% | 54-71-7 | 5g | $217 | 2024-03-01 | Buy |
| Sigma-Aldrich | P6503 | Pilocarpine hydrochloride ≥98% (titration), powder | 54-71-7 | 5g | $241 | 2024-03-01 | Buy |
(+)-Pilocarpine hydrochloride Chemical Properties,Uses,Production
Description
It is a bioactive chemical usually used as pharmaceutical agents such as acetylcholine receptor agonist, antiglaucoma agent, moitic, and sialogogue. Specifically, due to its desirable activity as acetylcholine receptor agonist, this chemical has been introduced to regulate muscarinic acetylcholine receptor function in acetylcholinesterase knockout mice.1 Moreover, because of its good antiglaucoma effect, this substance is applied in a timolol plus maximum-tolerated antiglaucoma therapy.2 In addition, this chemical has been demonstrated to show a favourable miotic activity when incorporated into polymer capsules.3 Besides, this compound can be utilized to relieve the xerostomia due to chronic graft-versus-host disease or total-body irradiation after bone-marrow transplantation for hematologic malignancies.4
Reference
- Li, B.; Duysen, E. G.; Volpicelli-Daley, L. A.; Levey, A. I.; Lockridge, O., Regulation of muscarinic acetylcholine receptor function in acetylcholinesterase knockout mice. Pharmacol. Biochem. Behav. 2003, 74, 977-986.
- Zimmerman, T. J.; Gillespie, J. E.; Kass, M. A.; Yablonski, M. E.; Becker, B., TIMOLOL PLUS MAXIMUM-TOLERATED ANTI-GLAUCOMA THERAPY. Arch. Ophthalmol. 1979, 97, 278-279.
- V Vidmar; S Pepeljnjak; Jalsenjak, I., The in vivo evaluation of poly(lactic acid) microcapsules of pilocarpine hydrochloride. Journal of Microencapsulation 1985, 2, 289-292.
- Nagler, R. M.; Nagler, A., Pilocarpine hydrochloride relieves xerostomia in chronic graft-versus-host disease: a sialometrical study. Bone Marrow Transplant. 1999, 23, 1007-1011.
Chemical Properties
Crystalline Solid
Originator
Andre Carpine, Andre Laboratries Pvt. Ltd., India
Uses
(+)-Pilocarpine hydrochloride is a muscarinic M1 and M2 acetylcholine receptor agonist with pKB values of 5 and 3.7, respectively. Systemic administration of (+)-Pilocarpine hydrochloride is typically used as an animal model for temporal lobe epilepsy and can mimic the generation of complex partial seizures by producing changes in hippocampal neuron morphology, membrane properties, and synaptic responses.
Uses
Antiglaucoma agent; miotic; sialogogue.
Definition
ChEBI: Pilocarpine hydrochloride is the hydrochloride salt of (+)-pilocarpine, a medication used to treat increased pressure inside the eye and dry mouth. It contains a (+)-pilocarpine.
Manufacturing Process
The 1-methylimidazole-5-aldehyde is easily accessible from sarcosine methyl ester hydrochloride and dimethylamino-2-azaprop-2-en-1
Pilocarpine ylidenedimethylammonium.
0.14 mol of ethyl diethylphosphonoethoxyacetate is slowly added dropwise with stirring and under inert gas to a suspension of 0.14 mol of NaH (paraffinfree) in 250 ml of abs. THF, the mixture is stirred for 1 h at 20°C and a solution of 0.093 mol of 1-methylimidazole-5-aldehyde in 100 ml of abs. THF is added dropwise. After stirring at 20°C for 10 min, the solvent is distilled off in vacuo, the residue is taken up in a little H2O, and the solution is acidified with 1 N HCl and washed several times with ether. The aqueous phase is rendered alkaline using 2 N NaOH with cooling (0°-5°C) and extracted several times with CH2Cl2. After drying of the organic extracts with Na2SO4, the solvent is removed in vacuo and 2-ethoxy-3-[(1-methyl-1H-imidazol-5yl)methyl]-acrilic acid ethyl ester. Yield: 99% of theory.
122 ml of 45% diisobutylaluminum hydride solution (328 mmol) are slowly added dropwise under inert gas, with stirring and ice cooling, to a solution of 137 mmol of 2-ethoxy-3-[(1-methyl-1H-imidazol-5-yl)methyl]-acrilic acid ethyl ester in 600 ml of abs. C6H6. Stirring of the mixture is continued for a further 30 min at 0°-5°C and 600 ml of CH3OH, then 100 ml of H2O, are slowly added. The hydroxide precipitate is filtered off with suction and washed several times with hot CH3OH. After drying of the combined filtrates the solvents are distilled off in vacuo and the residue is crystallized using C2H5OH. 2-ethoxy-3-[(1-methyl-1H-imidazol-5-yl)methyl]-prop-2-en-1-ol was obtained. Yield: 100% of theory. The crude product is pure enough for the subsequent reaction. Recrystallization of an analytical sample from CH3OH/acetone: melting point 129°C.
A solution of 58 mmol of 2-ethoxy-3-[(1-methyl-1H-imidazol-5-yl)methyl]prop-2-en-1-ol in 116.6 ml of HCl (= 116.6 mmol) is stirred at 30°-35°C for 1.5 h and concentrated in vacuo at the same temperature. The residual HCl is removed by distillation with CHCl3 in vacuo. After seeding, the residue crystallizes at 20°C (15 h) 1-hydroxy-3-[(1-methyl-1H-imidazol-5-yl)methyl]propan-2-one hydrochloride. The crystallizate is filtered off with suction, washed with a little CH3OH and dried in vacuo. Yield: 86% of theory; melting point 190°C.
About 80-90% of the equivalent amount of NaOCH3 solution in CH3OH is slowly added dropwise at 20°C with stirring and exclusion of moisture to a suspension of 21.24 mmol of 1-hydroxy-3-[(1-methyl-1H-imidazol-5yl)methyl]-propan-2-one hydrochloride in 80 ml of CH3OH, in the course of which the pH of 6.5 is not to be exceeded. The solvent is distilled off in vacuo at a maximum of 30°C and the residue of 1-hydroxy-3-[(1-methyl-1Himidazol-5-yl)methyl]-propan-2-one is purified by flash chromatography (silica gel; CHCl3/CH3OH). Yield: 100% of theory; viscous, orange-colored oil.
Catalytic amounts of 4-dimethylaminopyridine and a solution of 21.3 mmol of 1-hydroxy-3-[(1-methyl-1H-imidazol-5-yl)methyl]-propan-2-one in 80 ml of CH2Cl2 are added to a solution of 26.44 mmol of 2-diethylphosphonobutyric acid in 40 ml of purified CH2Cl2. After cooling to 0°-5°C, a solution of 23.5 mmol of dicyclohexylcarbodiimide in 60 ml of CH2Cl2 is added dropwise and the mixture is stirred for 1 h at 0°-5°C and for 2 h at 20°C. The crystallizeddicyclohexylurea is filtered off with suction and the filtrate is washed with H2O and saturated NaHCO3 solution. After drying of the organic phase the solvent is distilled off at 30°C in vacuo and the residue of 2-diethoxy-phosphoryl)butyric acid 3-[(1-methyl-1H-imidazol-5-yl)methyl]-2-oxo-propyl ester is purified by flash chromatography (silica gel; ethyl acetate/CH3OH). Yield: 95% of theory of a viscous, orange-colored oil.
A mixture of 5 mmol each of 80% NaH and 15-crown-5 in 50 ml of absol. toluene is stirred at 20°C under inert gas for 10 min and a solution of 5 mmol of 2-diethoxy-phosphoryl)-butiric acid 3-[(1-methyl-1H-imidazol-5-yl)methyl]2-oxo-propyl ester in 50 ml of absol. toluene is then added dropwise. Stirring is continued for a further 15 min under inert gas and the mixture is hydrolyzed with a little water until phase separation is detectable. After separating off the organic phase, the aqueous layer is saturated with NaCl and extracted several times with CHCl3. The combined organic phases are the solvent is distilled off at 40°C in vacuo and the residue 3-ethyl-4-[(1-methyl1H-imidazol-5-yl)methyl]-5H-furan-2-one is purified twice by flash chromatography (silica gel; ethyl acetate/CH3OH). Yield: 52% of theory; virtually colorless oil.
1.36 mmol of 3-ethyl-4-[(1-methyl-1H-imidazol-5-yl)methyl]-5H-furan-2-one in 15.5 ml of CH3OH are hydrogenated for 5 h at 50 bar and 60°C using 210 mg of Pd/carbon (10%). After filtering off the catalyst and distilling off the solvent at 30°C in vacuo, the oily residue (about 250 mg) is treated with 10 ml of 1 N HCl and the mixture is stirred for 3 h at 20°C. The hydrochloric acid is distilled off in vacuo at 35°-40°C, the oily residue is taken up in a little CH3OH and ether is added. The precipitate of pilocarpine hydrochloride is recrystallized from CH3OH/ether. Yield: 73% of theory; melting point 210°C.
brand name
Pilopine (Alcon); Salagen (Millot Laboratories, France).
Therapeutic Function
Cholinergic
General Description
Pilocarpine monohydrochlorideis the hydrochloride of an alkaloid obtainedfrom the dried leaflets of Pilocarpus jaborandi or P. microphyllus,in which it occurs to the extent of about 0.5% togetherwith other alkaloids.
Biological Activity
Muscarinic agonist.
Biochem/physiol Actions
Pilocarpine is alkaloid isolated from the shrub Pilocarpus species. It comprises imidazole ring and γ- lactone rings?in its structure. It has cholinergic potential and it stimulates the parasympathetic system. Pilocarpine is also effective for treating glaucoma. It stimulates ciliary muscle contraction by binding to muscarinic M3 receptors. It is effective in treating corneal dryness in Sjogren′s syndrome. It is a secretagogue, which improves salivary secretions in Xerostomia or a dry mouth condition in Sjogren′s syndrome patients and in radiotherapy patients with head and neck carcinoma.
Clinical Use
Pilocarpine, the salt of an alkaloid obtained from Pilocarpus jaborandi, is an example of a muscarinic agonist that does not adhere to the
traditional SAR. In 1876, Langley reported that extracts containing the alkaloid stimulated the end organs of parasympathetic neurons. The
structure of pilocarpine was reported in 1901.
Pilocarpine is marketed as tablets (Salogen), an ophthalmic solution, and gel. It penetrates the eye well and is the miotic of choice for
open-angle glaucoma and to terminate acute angle closure attacks. It also is used for the treatment of xerostomia (dryness of the mouth) caused
by radiation therapy of the head and neck, Sjogren's syndrome, or as a side effect of some psychotropic drugs.
Safety Profile
Poison by ingestion, intraperitoneal, and intravenous routes. Experimental teratogenic and reproductive effects. Human systemic effects: cardiac changes. When heated to decomposition it emits very toxic fumes of HCl and NOx. See also PILOCARPINE.
Veterinary Drugs and Treatments
Pilocarpine is a miotic agent that is rarely used in the treatment
of canine primary glaucoma. Pilocarpine causes the ciliary body
muscle to constrict placing posteriorly directed tension on the base
of the iris to mechanically pull open the iridocorneal angle structures.
By causing miosis, it may prevent closure of the iridocorneal
angle by preventing excess iris tissue from peripherally compromising
the outflow of aqueous humor. Pilocarpine has also been used
for diagnostic localization of parasympathetic denervation of the
iris sphincter caused by lesions or trauma to Cranial Nerve III.
The popularity of treatment of KCS with ophthalmic cyclosporine
and tacrolimus has been associated with a decline in the
use of pilocarpine for this disease; however, pilocarpine is still used
orally as the primary treatment of neurogenic keratoconjunctivitis
sicca in dogs as this condition does not respond to cyclosporine or
tacrolimus.
storage
Desiccate at RT
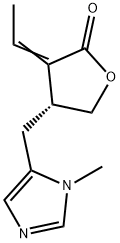
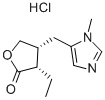
(+)-Pilocarpine hydrochloride Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Eaglech(shanghai)Pharmaceutical Co., Ltd | +8613884467586 | info@distant-chem.com | China | 10 | 58 |
| Hangzhou ICH Biofarm Co., Ltd | +undefined8613073685410 | sales@ichemie.com | China | 985 | 58 |
| Shaanxi Haibo Biotechnology Co., Ltd | +undefined18602966907 | qinhe02@xaltbio.com | China | 1000 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 | zheyansh@163.com | CHINA | 3620 | 58 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Chengdu GLP biotechnology Co Ltd | 028-87075086 13350802083 | scglp@glp-china.com | CHINA | 1824 | 58 |
| Biochempartner | 0086-13720134139 | candy@biochempartner.com | CHINA | 967 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
View Lastest Price from (+)-Pilocarpine hydrochloride manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-12 | (+)-Pilocarpine hydrochloride
54-71-7
|
US $0.00 / kg | 1kg | 99% | 2000ton | Shaanxi Haibo Biotechnology Co., Ltd | |
 |
2024-03-17 | (+)-Pilocarpine Hydrochloride
54-71-7
|
US $0.00-0.00 / g | 10g | 98% | 1000g | Eaglech pharmaceutical co.,ltd | |
 |
2023-11-01 | (+)-Pilocarpine hydrochloride
54-71-7
|
US $0.00-0.00 / kg | 1kg | 99% HPLC | 1000kg | Hangzhou ICH Biofarm Co., Ltd |
-

- (+)-Pilocarpine hydrochloride
54-71-7
- US $0.00 / kg
- 99%
- Shaanxi Haibo Biotechnology Co., Ltd
-

- (+)-Pilocarpine Hydrochloride
54-71-7
- US $0.00-0.00 / g
- 98%
- Eaglech pharmaceutical co.,ltd
-

- (+)-Pilocarpine hydrochloride
54-71-7
- US $0.00-0.00 / kg
- 99% HPLC
- Hangzhou ICH Biofarm Co., Ltd





