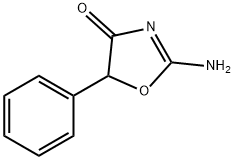
Pemoline
- CAS No.
- 2152-34-3
- Chemical Name:
- Pemoline
- Synonyms
- PIO;yh1;Fio;Yh 1;npl1;pt360;Ronyl;Sofro;cs293;H 310
- CBNumber:
- CB4180793
- Molecular Formula:
- C9H8N2O2
- Molecular Weight:
- 176.17
- MDL Number:
- MFCD00083197
- MOL File:
- 2152-34-3.mol
| Melting point | 255-256°C |
|---|---|
| Boiling point | 307.77°C (rough estimate) |
| Density | 1.2662 (rough estimate) |
| refractive index | 1.5570 (estimate) |
| storage temp. | Store at RT |
| solubility | DMSO: soluble28mg/mL |
| pka | pKa 10.5 (Uncertain) |
| form | solid |
| color | white |
| CAS DataBase Reference | 2152-34-3(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | 7GAQ2332NK |
| ATC code | N06BA05 |
| NIST Chemistry Reference | 4(5H)-oxazolone, 2-amino-5-phenyl-(2152-34-3) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302+H312+H332 |
| Precautionary statements | P261-P264-P280-P301+P312-P302+P352+P312-P304+P340+P312 |
| Hazard Codes | Xn,C,F,T |
| Risk Statements | 22-34-11-20/21/22 |
| Safety Statements | 16-26-36/37/39-45-36/37-36 |
| WGK Germany | 3 |
| RTECS | PB8750000 |
| Toxicity | LD50 orally in rats: 500 mg/kg (Schafer) |
Pemoline Chemical Properties,Uses,Production
Chemical Properties
White Solid
Originator
Deltamine,Aron,France,1960
Uses
A CNS stimulant. Controlled Substance
Definition
ChEBI: A member of the class of 1,3-oxazoles that is 1,3-oxazol-4(5H)-one which is substituted by an amino group at position 2 and by a phenyl group at position 5. A central nervous system stimulant, it was used to treat hyperactivity disorders in children, but withdrawn from use following reports of serious hepatotoxicity.
Manufacturing Process
It is preferably prepared by reacting mandelic acid ethyl ester with guanidine in boiling alcoholic solution whereby it is obtained as difficultly soluble precipitate with a yield of 90%.
This compound is a white, crystalline compound melting at 256°-257°C with decomposition. It is readily soluble in concentrated aqueous alkali hydroxide solutions and in concentrated aqueous mineral acids.
brand name
Cylert (Abbott).
Therapeutic Function
Psychostimulant
World Health Organization (WHO)
Pemoline was introduced in 1975 for the treatment of attentiondeficit disorder. Because of its central stimulating effects it has also been used in weight control in combination with anorectic agents, laxatives.
General Description
Pemoline, 2-amino-5-phenyl-4(5H)-oxazolone (Cylert), hasa unique structure.
The compound is described as having an overall effect onthe CNS like that of methylphenidate. Pemoline requires 3to 4 weeks of administration, however, to take effect. A partialexplanation for the delayed effect may be that one of theactions of the agent, as observed in rats, is to increase therate of synthesis of DA.
Biological Activity
Long-acting central stimulant that induces self-injurious behavior in rats. Acts as an indirect monoamine agonist.
Pemoline Preparation Products And Raw materials
Raw materials
1of2