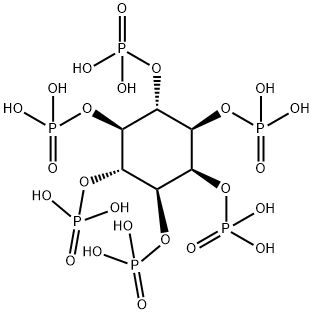
Phytic acid
- CAS No.
- 83-86-3
- Chemical Name:
- Phytic acid
- Synonyms
- INOSITOL HEXAPHOSPHATE;phytic;phyticacidsolution;myo-Inositol hexakisphosphate;alkovert;fyticacid;PHYTIC ACID POWDER;INOSITOL HEXAPHOSPHORIC ACID;WHL;RP 3000
- CBNumber:
- CB4321770
- Molecular Formula:
- C6H18O24P6
- Molecular Weight:
- 660.04
- MDL Number:
- MFCD00082309
- MOL File:
- 83-86-3.mol
- MSDS File:
- SDS
| Melting point | <25℃ |
|---|---|
| Boiling point | 105 °C |
| Density | 1.432 g/mL at 25 °C |
| vapor pressure | 0.039Pa at 60℃ |
| refractive index |
n |
| storage temp. | 2-8°C |
| solubility | Acetone (Slightly), Methanol (Slightly), Water (Soluble) |
| form | Colourless to Light Brown Solution |
| pka | 1.13±0.10(Predicted) |
| Specific Gravity | 1.282 |
| Odor | odorless |
| Water Solubility | MISCIBLE |
| Merck | 14,7387 |
| BRN | 2201952 |
| Stability | Stable. Incompatible with strong oxidizing agents. |
| InChIKey | IMQLKJBTEOYOSI-GPIVLXJGSA-N |
| CAS DataBase Reference | 83-86-3(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| NCI Dictionary of Cancer Terms | inositol hexaphosphate; phytic acid |
| FDA UNII | 7IGF0S7R8I |
| EPA Substance Registry System | myo-Inositol, hexakis(dihydrogen phosphate) (83-86-3) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS05,GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H290-H302-H314 | |||||||||
| Precautionary statements | P234-P270-P280-P301+P312-P303+P361+P353-P305+P351+P338 | |||||||||
| Hazard Codes | Xi,C | |||||||||
| Risk Statements | 36/37/38-35 | |||||||||
| Safety Statements | 26-36-37/39-45-36/37/39 | |||||||||
| RIDADR | 1760 | |||||||||
| WGK Germany | - | |||||||||
| RTECS | NM7525000 | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29199000 | |||||||||
| Toxicity | LD50 intravenous in mouse: 500mg/kg | |||||||||
| NFPA 704 |
|
Phytic acid price More Price(32)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 593648 | Phytic acid solution 50 % (w/w) in H2O | 83-86-3 | 1l | $189 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1537910 | Phytic acid solution United States Pharmacopeia (USP) Reference Standard | 83-86-3 | 5ml | $441 | 2024-03-01 | Buy |
| TCI Chemical | P0409 | Phytic Acid (ca. 50% in Water, ca. 1.1mol/L) | 83-86-3 | 25g | $23 | 2024-03-01 | Buy |
| TCI Chemical | P0409 | Phytic Acid (ca. 50% in Water, ca. 1.1mol/L) | 83-86-3 | 500g | $87 | 2024-03-01 | Buy |
| Sigma-Aldrich | 593648 | Phytic acid solution 50 % (w/w) in H2O | 83-86-3 | 250ml | $72.2 | 2024-03-01 | Buy |
Phytic acid Chemical Properties,Uses,Production
General Information
Phytic acid (PA, molecular formula: C6H18O24P6, molecular structure is as follows ), also known as inositol hexakisphosphate, hexaphosphoinositol, myo-inositol hexakisphosphate, IP6, is pale yellow to pale brown slurry liquid, soluble in water, ethanol and acetone, nearly insoluble in ether, benzene and chloroform. It is stable and incompatible with strong oxidizing agents[1][2][3]. It’s basically non-toxic and should be sealed in a cool and dry place. Storage and transportation can be according to general chemical regulations.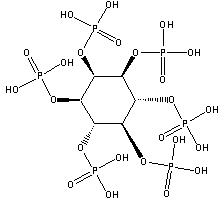
PA first identified in 1855[4] is a naturally occurring compound formed during the maturation of seeds and cereal grains. It’s the storage form of phosphorus, an important mineral used in the production of energy as well as the formation of structural elements like cell membranes[5]. In the seeds of legumes, it accounts for about 70% of the phosphate content and is structurally integrated with the protein bodies as phytin, a mixed potassium, magnesium, and calcium salt of inositol[6].
PA is the most abundant form of phosphorus in plants. During food processing and digestion, inositol hexaphosphate can be partially dephosphorylated to produce degradation products, such as penta-, tetra-, and triphosphate, by the action of endogenous phytases, which are found in most PA-containing seeds from higher plants. Seed germination results in increased phytase activity, and PA hydrolysis releases phosphate and free myoinositol for use during plant development[6].
PA has the ability to bind minerals, proteins, and starch (either indirectly or directly). This binding alters the solubility, functionality, digestion, and absorption of these food components. At normal pH range, the phosphate groups of phytic acid are negatively charged, allowing interaction with positively charged components such as minerals and proteins. Metal ions may bind with one or more phosphate groups forming complexes of varying solubility. Proteins are able to bind directly with PA through electrostatic charges. Starch binding may also occur via hydrogen bond formation. Zinc appears to be the most affected by PA because it forms the most stable and insoluble complex. Other minerals and nutrients that are affected include calcium(Ca), sodium (Na), iron (Fe), magnesium (Mg), manganese (Mn), and chlorine (Cl)[4].
Preparation and Analysis
Now, there are many preparation methods for PA, which can be summarized as two main types: chemical synthesis and extraction.
The principle of chemical synthesis in every experiment has been to obtain the desired product by heating together inosite and orthophosphoric acid[7]. But this method has no industrial significance.
Extraction method which extracts phytic acid from natural plants has actual industrial significance currently. PA is generally extracted from agricultural and sideline products such as rice bran and plant germ. At present, the main extraction methods are solvent extraction assisted method, microwave assisted extraction method, ultrasonic assisted extraction method, membrane separation assist method, etc.
In addition, the adsorption method which uses an anion exchange resin to adsorb the phytic acid in the extract, and then elutes the adsorbed phytic acid with an eluent to obtain a phytate is another way to prepare phytic acid.
Most analytical methods are based on extraction or isolation of PA.
The AOAC anion-exchange method is one that has been used to estimate phytic acid content in products. However, this method is not capable of distinguishing phytic acid (IP6) from other inositol phosphates (IP5–IP1). This analytical method groups all of these components together and the result is often an overestimation of the amount of phytic acid present.
The high-performance liquid chromatography (HPLC) method is the primary means of separation and quantification. HPLC is capable of separating phytic acid and inositol phosphates as separate entities. It also has the sensitivity and reproducibility to measure low concentrations in products. However the reagents used in this method must be pure and free from metals or it will cause distortion in the readings[4].
Nutrition
Along with saponins and lectins, phytic acid is considered an anti-nutrient[5][8].
PA in plant-derived foods we have can lead to poor bioavailability (BV) of minerals due to its strong ability to chelate multivalent metal ions, especially zinc, calcium, and iron which can form very insoluble salts that are poorly absorbed from the gastrointestinal tract[9]. Because humans don't have the enzymes needed to break down phytates, as much as 50 percent of these minerals -especially iron -passes out of the body unabsorbed[10]. While phytic acid not only grabs on to or chelates important minerals, but also inhibits enzymes that we need to digest our food, including pepsin needed for the breakdown of proteins in the stomach, and amylase needed for the breakdown of starch into sugar. Trypsin needed for protein digestion in the small intestine is also inhibited by phytates[11].
But fortunately, several preparation methods: soaking, sprouting, fermentation, can significantly reduce the phytic acid content of foods. Cereals and legumes are often soaked in water overnight to reduce their phytate content. The sprouting of seeds, grains and legumes, also known as germination, causes phytate degradation. Organic acids, formed during fermentation, promote phytate breakdown. Lactic acid fermentation is the preferred method, a good example of which is the making of sourdough. Combining these methods can reduce phytate content substantially[9].
Although offering some negative effects, PA actually has some potential healthful effects, such as in lowering serum cholesterol and triglycerides, and preventing heart disease, renal stone formation, and certain types of cancer, such as colon cancer. The metabolites of PA may also function as second messengers, whereas the naturally occurring compounds have been suggested to act as neuromodulators. PA is also considered to be a natural antioxidant and is suggested to have potential functions of reducing lipid peroxidation[6].
Applications
The most significant feature of PA is its strong complexation with metal ions and oxidation resistance[1][12], which makes it widely used.
In the food industry
Adding 0.05% to 0.1% phytic acid and sodium phytate to beverages and alcohols can remove the calcium, iron and copper heavy metal elements in beverages and alcohols which can protect the human body.
The preservative prepared with phytic acid sprayed on fruits and vegetables can effectively improve the fresh-keeping period. Proper addition of phytic acid as an antioxidant to vegetable oils or high-oil foods can extend the shelf life by 3 to 5 times.
Adding phytic acid to canned foods can achieve a stable color protection effect. Adding a trace amount of phytic acid to canned fish, shrimp, squid and other aquatic products can prevent the formation of struvite (glass-like ammonium magnesium phosphate crystal).
In the pharmaceutical industry
PA itself is a beneficial nutrient for the human body, which can promote the release of oxygen in oxyhemoglobin, improve the function of red blood cells, and prolong the survival of red blood cells. In addition, phytic acid is hydrolyzed in the human body to produce inositol and phospholipids. The former has anti-aging effects, and the latter is an important component of human cells. Phytic acid can relieve lead poisoning and can be used as a preventive agent for heavy metal poisoning. It can also prevent cancer and heart disease and can inhibit colon cancer and early breast cancer. Phytic acid can bind to iron in the intestine which could reduce the free radical production and inhibit cancer[5][10].
In other industries
PA can complex with metal ions bound to the surface of magnesium alloy to form a conversion coating, which can improve the resistance of magnesium alloy towards corrosion[13].
PA is a heat and light stabilizer, a flame retardant and an antistatic agent. The incorporation of a trace amount of phytic acid in the resin can maintain light and heat stability for a long time, and can effectively prevent self-agglomeration. It can be used as a hydrogen peroxide storage stabilizer as it can prevent the decomposition of hydrogen peroxide. It can also be used as an antistatic agent for liquid fuels and fibers, as an anti-explosive additive for aviation gasoline, and as an excellent flame retardant for cotton, polyester, and silk fabrics.
Reference
- https://www.chemicalbook.com/ChemicalProductProperty_EN_CB4321770.htm
- http://www.chemnet.com/ChinaSuppliers/26279/Phytic-acid--823005.html
- https://www.encyclopedia.com/sports-and-everyday-life/food-and-drink/food-and-cooking/phytic-acid
- Lori Oatway, Thava Vasanthan, James H. Helm. Phytic Acid[J]. Food Reviews International, 2001, 17(4): 419-431
- http://breakingmuscle.com/healthy-eating/dissecting-anti-nutrients-the-good-and-bad-of-phytic-acid
- Jin R. Zhou, John W. Erdman Jr. Phytic Acid in Health and Disease[J]. Critical Reviews in Food Science and Nutrition, 1995, 35(6): 495-508
- R. J. Anderson. Synthesis of Phytic Acid[J]. Journal of Biological Chemistry, 1920, 43:117-128
- http://www.nourishingdays.com/2010/09/what-is-phytic-acid/
- https://www.healthline.com/nutrition/phytic-acid-101#section5
- https://www.livestrong.com/article/281955-foods-containing-phytic-acid/
- https://www.westonaprice.org/health-topics/vegetarianism-and-plant-foods/living-with-phytic-acid/
- http://www.yourdictionary.com/phytic-acid
- https://www.sigmaaldrich.com/catalog/product/aldrich/593648?lang=en®ion=HK
Chemical Properties
colourless to pale yellow liquid
Originator
Rencal, Squibb, US ,1962
Uses
A compound which inhibits cell differentiation and cell proliferation.
Uses
phytic acid is used to help maintain product stability. Its therapeutic activities are said to include skin-lightening, anti-inflammatory and anti-oxidant properties. It is naturally occurring in grains, seeds, and beans.
Uses
The principal storage form of phosphorus in many plant tissues, especially bran and seeds. It has antioxidant effect as well as neuroprotective effect by chelating iron. Inositol hexakisphosphate kina se type 2 (InsP6K2), which converts phytic acid to inositol pyrophosphate diphosphoinositol pentakisphosphate, is believed to induce pathogenesis of Huntington disease (HD).
Definition
ChEBI: A myo-inositol hexakisphosphate in which each hydroxy group of myo-inositol is monophosphorylated.
Manufacturing Process
Cereal grains are particularly rich in phytates; corn steep water produced in the wet milling of corn, is one of the best sources of such material. To recover the phytate from corn steep water it is customary to neutralize the same withan alkaline material, suitably lime, causing the phytate to precipitate as a crude salt which can be removed readily by filtration. This material contains substantial amounts of magnesium, even though lime may have been employed as precipitant, and traces of other metallic ions, as well as some proteinaceous materials and other contaminants from the steep water. It may be partially purified by dissolving in acid and reprecipitating but, nevertheless, such commercial phytates do not represent pure salts. They always contain some magnesium, appreciable amounts of iron and nitrogenous materials, and traces of heavy metals, such as copper.
Heretofore, no economical method for preparing pure phytic acid was known. The classical method was to dissolve calcium phytate in an acid such as hydrochloric acid, and then add a solution of a copper salt, such as copper sulfate to precipitate copper phytate. The latter was suspended in water and treated with hydrogen sulfide, which formed insoluble copper sulfide and released phytic acid to the solution. After removing the copper sulfide by filtration, the filtrate was concentrated to yield phytic acid as a syrup.
The phytic acid in the form of a calcium phytate press cake may however be contacted with a cation exchange resin to replace the calcium with sodium to yield phytate sodium.
Therapeutic Function
Hypocalcemic
General Description
Phytic acid is a mineral chelator that can bind to minerals to form mineral-phytate complex.It can also complex with metal ions bound to the surface of magnesium alloy to form a conversion coating, which can improve the resistance of magnesium alloy towards corrosion.
Agricultural Uses
Phytic acid is inositol hexaphosphoric acid which exists in several stereoisomers. It is a source of phosphorus compounds for seeds and young plants.
Safety Profile
Poison by intravenous route. When heated to decomposition it emits toxic fumes of POx.
Phytic acid Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| ENBRIDGE PHARMTECH CO., LTD. | +8613812269233 | tinayang@enbridgepharm.com | China | 303 | 58 |
| Hubei Langyou International Trading Co., Ltd | +8618874586545 | linda@xrdchem.cn | China | 197 | 58 |
| Hebei Saisier Technology Co., LTD | +86-18400010335 +86-13102810335 | admin@hbsaisier.cn | China | 747 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9348 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 | info@tnjchem.com | China | 2989 | 55 |
| Shanxi Naipu Import and Export Co.,Ltd | +86-13734021967 +8613734021967 | kaia@neputrading.com | China | 1011 | 58 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 | zheyansh@163.com | CHINA | 3620 | 58 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
View Lastest Price from Phytic acid manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-26 | Phytic acid
83-86-3
|
US $0.00 / Kg/Drum | 1KG | ≥50% | 500mt/year | Jinan Finer Chemical Co., Ltd | |
 |
2024-04-26 | Phytic acid
83-86-3
|
US $16.00 / KG | 5KG | 99% | 20t | Hebei Yime New Material Technology Co., Ltd. | |
 |
2024-04-26 | Phytic acid
83-86-3
|
US $16.00 / KG | 5KG | 50% | 20t | Hebei Yime New Material Technology Co., Ltd. |
-

- Phytic acid
83-86-3
- US $0.00 / Kg/Drum
- ≥50%
- Jinan Finer Chemical Co., Ltd
-

- Phytic acid
83-86-3
- US $16.00 / KG
- 99%
- Hebei Yime New Material Technology Co., Ltd.
-

- Phytic acid
83-86-3
- US $16.00 / KG
- 50%
- Hebei Yime New Material Technology Co., Ltd.
83-86-3(Phytic acid)Related Search:
1of4