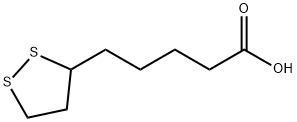
α-Lipoic Acid
- CAS No.
- 1077-28-7
- Chemical Name:
- α-Lipoic Acid
- Synonyms
- ALA;THIOCTIC ACID;ALPHA-LIPOIC ACID;ALPHA-LIPOIC ACID;DL-THIOCTIC ACID;Thioctic acid CRS;A-LIPOIC ACID;alpha lipoic acid powder;DL-A-LIPOIC ACID;Alpha Acid Lipoic
- CBNumber:
- CB5180699
- Molecular Formula:
- C8H14O2S2
- Molecular Weight:
- 206.33
- MDL Number:
- MFCD00005474
- MOL File:
- 1077-28-7.mol
- MSDS File:
- SDS
| Melting point | 60-62 °C |
|---|---|
| Boiling point | 160-165 °C(lit.) |
| Density | 1.2888 (rough estimate) |
| refractive index | 1.5200 (estimate) |
| Flash point | 160-165°C |
| storage temp. | 2-8°C |
| solubility | ethanol: 50 mg/mL |
| form | Crystals or Crystalline Powder |
| pka | 4.75±0.10(Predicted) |
| color | Yellow |
| Water Solubility | 0.9 g/L (20 ºC) |
| Merck | 14,9326 |
| BRN | 81853 |
| Stability | Stable. Incompatible with strong oxidizing agents. |
| InChIKey | AGBQKNBQESQNJD-UHFFFAOYSA-N |
| LogP | 2.530 (est) |
| CAS DataBase Reference | 1077-28-7(CAS DataBase Reference) |
| FDA UNII | 73Y7P0K73Y |
| NIST Chemistry Reference | Dl-thioctic acid(1077-28-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302-H315-H317-H319-H411 | |||||||||
| Precautionary statements | P261-P273-P280-P301+P312-P302+P352-P305+P351+P338 | |||||||||
| Hazard Codes | Xn | |||||||||
| Risk Statements | 22-20/21/22 | |||||||||
| Safety Statements | 37/39-26-24/25-36-36/37/39 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | JP1192000 | |||||||||
| HS Code | 29349990 | |||||||||
| NFPA 704 |
|
α-Lipoic Acid price More Price(61)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 62320 | (±)-α-Lipoic acid ≥98.0% | 1077-28-7 | 5G | $112 | 2024-03-01 | Buy |
| Sigma-Aldrich | PHR2561 | Alpha Lipoic Acid | 1077-28-7 | 1G | $173 | 2024-03-01 | Buy |
| Sigma-Aldrich | 62320 | (±)-α-Lipoic acid ≥98.0% | 1077-28-7 | 25g | $286 | 2024-03-01 | Buy |
| Sigma-Aldrich | 437692 | α-Lipoic Acid - CAS 1077-28-7 - Calbiochem α-Lipoic Acid, CAS 1077-28-7 is an oxidized form with antioxidant properties. Reduces renal lipid peroxidation in rats with cisplatin n-induced nephrotoxicity. | 1077-28-7 | 5G | $111 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1368201 | Alpha Lipoic Acid United States Pharmacopeia (USP) Reference Standard | 1077-28-7 | 500mg | $394 | 2024-03-01 | Buy |
α-Lipoic Acid Chemical Properties,Uses,Production
Description
DL-
Chemical Properties
Light-Yellow Solid
Originator
Neurium,Hexal
Uses
Lipoic acid [CAS: 1077-28-7] (6,8-dimercaptooctanoic acid), a lipophilic endogenous disulfide that can be reduced to the dithiol dihydrolipoic acid, protects against free-radical-mediated injury both in vivo and in vitro.
Uses
thioctic acid is also known as alpha lipoic acid. It is an anti-oxidant.
Uses
A fat-metabolism stimulator
Uses
(±)-α-Lipoic acid has been used in in vitro lipoylation studies and in the pyruvate dehydrogenase complex (PDC)-pyruvate dehydrogenase kinase (PDHK) functional assay. It has also been used to investigate its antioxidative effect on developing cerebellum of rats exposed to arsenic during postnatal period.
Definition
ChEBI: Lipoic acid is a heterocyclic thia fatty acid comprising pentanoic acid with a 1,2-dithiolan-3-yl group at the 5-position. It has a role as a fundamental metabolite and a geroprotector. It is a member of dithiolanes, a heterocyclic fatty acid and a thia fatty acid. It is functionally related to an octanoic acid. It is a conjugate acid of a lipoate.
Manufacturing Process
To a suspension of 106 g of anhydrous aluminum chloride in 450 ml of
carbontetrachloride is added dropwise, with vigorous stirring, 70 g of ethyl 8-
chloroformylvalerate (H. Bergs, C. Wittfeld and H. Frank, Ber., 67B, 1622
(1947)). The temperature is maintained at 25°C. The cooling bath is removed
and ethylene is passed in for a period of 2 hours. The reaction mixture is
poured onto cracked ice, the organic layer separated, and the aqueous layer
extracted with 200 ml of chloroform. The combined organic extracts are dried
over anhydrous sodium sulfate and the solvent removed in vacuo. The dark
colored oil remaining, crude ethyl 8-chloro-6-oxooctanoate is distilled in vacuo
through a 6 in. Vigreaux column. After small forerun, the main fraction, 48-54
g (72-80%), B.P. 112-114°C (2 mm.); n25
D1-4485, is collected.
Redistilled thiolacetic acid (14.7 g) is cooled in an ice-bath and neutralized to
the phenolphthalein end-point with a 10% solution of potassium hydroxide in
ethanol (approximately 135 ml required). To this solution is added 29 g of
ethyl-6,8-dibromooctanoate and the mixture is stirred and heated under reflux
in an atmosphere of nitrogen for 5 hours. The reaction mixture, which
contains ethyl 6,8-diacetyl-mercaptooctanoate, is cooled and 35 g of
potassium hydroxide (85%) is added. The reaction mixture is stirred at room
temperature in an atmosphere of nitrogen for 17 hours, then acidified (pH less
than 1) with 6 N hydrochloric acid. Ethanol is removed in vacuo, sufficient
water is added to dissolve the inorganic solids and the mixture is extracted
with two 150 ml portions of chloroform. To the combined organic extracts,
which contain 6,8-dimercaptooctanoic acid , is added 575 ml of chloroform
and 210 ml of water. This mixture is stirred vigorously in an atmosphere of
nitrogen While sufficient iodoform reagent (R. L. Shriner and R. C. Fuson,
"Identification of Organic Compounds," 2nd. Ed., John Wiley and Sons, New
York, N.Y., 1940, p. 53) is added dropwise during a 6 hour period to give a
permanent brown color. Approximately 185 ml of iodoform reagent is
required. The organic layer is separated, washed with 500 ml of 1% sodium
thiosulfate solution, and then extracted with two 250 ml portions of 5%
sodium bicarbonate solution. The aqueous extracts are acidified (pH less than
1) with 6 N hydrochloric acid and extarcted with two 125 ml portions of
chloroform. The combined chloroform extracts are dried over anhydrous
sodium sulfate and the solvent is then removed in vacuo. The yellow viscous
oil remaining solidifies when cooled and scratched. This solid material is
extracted with three 300 ml portions of boiling Skelly B solvent (essentially nhexane).
The combined extracts are seeded with crystalline DL-α-lipoic acid
and allowed to stand at room temperature overnight and then in a refrigerator
for several hours. Large yellow crystals separate, M.P. 60.5-61.5°C. The yield
of product is 10.8-12.3 g (60-68%). 1,2-Dithiolane-3-pentanoic acid was
recrystallized from Skelly B solvent, M.P. 61-62°C.
brand name
Liposan;Thioactacid.
Therapeutic Function
Growth factor, Lipotropic, Detoxicant
Synthesis Reference(s)
Journal of the American Chemical Society, 79, p. 6483, 1957 DOI: 10.1021/ja01581a033
General Description
Oxidized form. Has antioxidant properties. Growth factor for many bacteria and protozoa.
Flammability and Explosibility
Not classified
Pharmaceutical Applications
Lipoic acid(ALA), also known as α-lipoic acid (alpha-lipoic acid) or thioctic acid, has the formula C8H14S2O2 and
systematic name 5-(1,2-dithiolan-3-yl)pentanoic acid. It contains a disulfide group, which can be transformed
in the body to a dithiol group.
ALA has been on the market since the 1950s as a dietary supplement. It is a natural antioxidant usually
made by the body. The advantage of ALA over other antioxidants such as vitamin C and E is that it is soluble
both in water and in fat. Researchers in the former Soviet Union found that ALA can chelate mercury
once it is transformed into the dithiol-containing compound. ALA can penetrate both the blood–brain barrier
and the cell membrane and therefore would be a very interesting chelating agent. Nevertheless, there is much
debate about its mode of action, side effects and effectiveness. Other antidotes, such as BAL and DMSA, are
more efficient in the removal of heavy metals. ALA has not received FDA approval as a chelating agent, but
it is still sold as a food supplement.
Biochem/physiol Actions
Antioxidant and coenzyme needed for the activity of enzyme complexes such as those of pyruvate dehydrogenase and glycine decarboxylase. Exogenous thioctic acid is reduced intracellularly by two or more enzymes. The reduced form then influences a number of cell processes by direct radical scavenging, recycling of other antioxidants, increasing glutathione synthesis, and modulating transcription factor activity particularly that of NF-κB. Decreases the phagocytosis of myelin by macrophages.
storage
Store at +4°C
Purification Methods
It forms yellow needles from cyclohexane or hexane and has been distilled at high vacuum; and sublimes at ~90o and very high vacuum. It is insoluble in H2O but dissolves in alkaline solution. [Lewis & Raphael J Chem Soc 4263 1962, Soper et al. J Am Chem Soc 76 4109, Reed & Niu J Am Chem Soc 77 416 1955, Tsuji et al. J Org Chem 43 3606 1978, Calvin Fed Proc USA 13 703 1954.] The S-benzylisothiuronium salt has m 153-154o (evacuated capillary, from MeOH), 132-134o, 135-137o (from EtOH). The dand lforms have m 45-47.5o and [] D 23 ±113o (c 1.88, *C6H6) and have UV in MeOH with max at 330nm ( 140). [Beilstein 19/7 V 237.] The reduced form, (±)-6,8-dimercaptooctanoic acid, [7516-48-5] M 208.3, is a light yellow liquid which is sold in sealed ampoules.
α-Lipoic Acid Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shandong Luning Pharmaceutical Co., Ltd. | 0546-6491488 +8613305469775 | China | 40 | 58 | |
| Xi'an Kono chem co., Ltd., | 029-86107037 13289246953 | info@konochemical.com | China | 2995 | 58 |
| Xi'an ZB Biotech Co.,Ltd | +8618591943808 | sales01@xazbbio.com | China | 816 | 58 |
| Guangzhou TongYi biochemistry technology Co.,LTD | +8613073028829 | mack@tongyon.com | China | 2996 | 58 |
| XI'AN TIANGUANGYUAN BIOTECH CO., LTD. | +86-029-86333380 18829239519 | sales06@tgybio.com | China | 959 | 58 |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 | info@gihichemicals.com | China | 49999 | 58 |
| Wuhan Quanjinci New Material Co.,Ltd. | +8615271838296 | kyra@quanjinci.com | China | 1532 | 58 |
| Chongqing Zhihe Biopharmaceutical Co., Ltd. | +86-18580541567 +86-17782035140 | sales@zhswyy.com | China | 338 | 58 |
| BINBO BIOLOGICAL CO.,LTD | +8618629063126 | info@binbobiological.com | China | 290 | 58 |
| Lihe Pharm Technology(Wuhan)Co.,Ltd | +8618071517867 | info@lihepharma.com | China | 194 | 58 |
Related articles
- α-Lipoic acid: pharmacokinetics and activities
- α-Lipoic acid exhibits a wide range of potential health benefits, including antioxidant activity, antidiabetic effects, and an....
- Jul 20,2023
- Systhesis of Lipoic acid
- α- Lipoic acid is light yellow powder crystal, almost tasteless Low solubility in water, about 1 g / L (20 ° C) Soluble in 10%....
- Dec 31,2021
- What is α- Lipoic acid?
- Alpha-Lipoic acid is an organic sulfur compound derived from caprylic acid, which is usually produced in animals and is essent....
- Aug 30,2021
View Lastest Price from α-Lipoic Acid manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-26 | α-Lipoic Acid
1077-28-7
|
US $0.00 / G | 1G | 99% | 20 | CONTIDE BIOTECH CO.,LTD | |
 |
2024-04-26 | α-Lipoic Acid
1077-28-7
|
US $0.00-0.00 / kgkg | 1kgkg | 99% | 3000kg | Jinan Jianfeng Chemical Co., Ltd | |
 |
2024-04-24 | α-Lipoic Acid
1077-28-7
|
US $0.00-0.00 / kg | 0.10000000kg | 99% | 2000KG | Chongqing Zhihe Biopharmaceutical Co., Ltd. |
-

- α-Lipoic Acid
1077-28-7
- US $0.00 / G
- 99%
- CONTIDE BIOTECH CO.,LTD
-

- α-Lipoic Acid
1077-28-7
- US $0.00-0.00 / kgkg
- 99%
- Jinan Jianfeng Chemical Co., Ltd
-

- α-Lipoic Acid
1077-28-7
- US $0.00-0.00 / kg
- 99%
- Chongqing Zhihe Biopharmaceutical Co., Ltd.