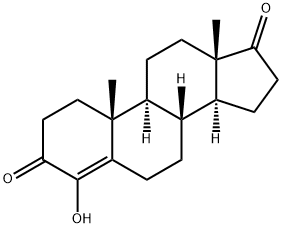
Formestane
- CAS No.
- 566-48-3
- Chemical Name:
- Formestane
- Synonyms
- 4-OHA;CYPXIX;LENTARON;CGP-32349;P-450AROM;FORMESTANE;FROMESTANE;NSC 282175;Stan FuMei;FORMESTAQNE
- CBNumber:
- CB6251183
- Molecular Formula:
- C19H26O3
- Molecular Weight:
- 302.41
- MDL Number:
- MFCD00057814
- MOL File:
- 566-48-3.mol
- MSDS File:
- SDS
| Melting point | 199-202°C |
|---|---|
| alpha | D20 +181° (c = 7.7 in chloroform) |
| Boiling point | 383.44°C (rough estimate) |
| Density | 1.1189 (rough estimate) |
| refractive index | 1.4200 (estimate) |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | solid |
| pka | 9.31±0.60(Predicted) |
| color | White to Off-White |
| InChI | InChI=1S/C19H26O3/c1-18-10-8-15(20)17(22)14(18)4-3-11-12-5-6-16(21)19(12,2)9-7-13(11)18/h11-13,22H,3-10H2,1-2H3/t11-,12-,13-,18+,19-/m0/s1 |
| InChIKey | OSVMTWJCGUFAOD-KZQROQTASA-N |
| SMILES | C1(=O)C(O)=C2[C@](C)(CC1)[C@]1([H])[C@]([H])([C@@]3([H])[C@@](CC1)(C)C(=O)CC3)CC2 |
| CAS DataBase Reference | 566-48-3(CAS DataBase Reference) |
| FDA UNII | PUB9T8T355 |
| NCI Drug Dictionary | formestane |
| ATC code | L02BG02 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|---|---|
| Signal word | Danger |
| Hazard statements | H360 |
| Precautionary statements | P201-P308+P313 |
| Hazard Codes | T |
| Risk Statements | 60 |
| Safety Statements | 53-36/37/39-45 |
| WGK Germany | 3 |
| RTECS | BV8152500 |
Formestane price More Price(18)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | SAB4500606 | Anti-Cytochrome P450 19A1 antibody produced in rabbit affinity isolated antibody | 566-48-3 | 100μG | $506 | 2024-03-01 | Buy |
| Sigma-Aldrich | F2552 | Formestane solid | 566-48-3 | 1g | $239 | 2024-03-01 | Buy |
| Cayman Chemical | 18282 | 4-hydroxy Androstenedione ≥98% | 566-48-3 | 100mg | $32 | 2024-03-01 | Buy |
| Cayman Chemical | 18282 | 4-hydroxy Androstenedione ≥98% | 566-48-3 | 1g | $184 | 2024-03-01 | Buy |
| Cayman Chemical | 18282 | 4-hydroxy Androstenedione ≥98% | 566-48-3 | 500mg | $116 | 2024-03-01 | Buy |
Formestane Chemical Properties,Uses,Production
Anti-cancer drugs
Formestane also known as lentaron,is an anti-cancer drug, it is primarily used for the treatment of postmenopausal women with advanced breast cancer, it is also effective in prostate cancer.
Formestane is a androstenedione derivative, and it belongs to an aromatase inhibitor with aminoglutethimide, it is a hormone antineoplastic agent. In physiological conditions, it may competitively inhibit the synthesis of the enzyme leading to estrogen biosynthesis decrease in tissues, then it plays its role in cancer. When the tumor tissue growth relies on the presence of estrogen, in order to inhibit tumor growth, the elimination of tumor estrogen-mediated growth stimulation is necessary. This product is more selective than aminoglutethimide while its activity is 100 to 1000 times of aminoglutethimide, and it does not inhibit the synthesis of adrenal hormones,without having to recharge cortisone, etc . The in vitro inhibition of aromatase enzyme of this product is 60 times stronger than aminoglutethimide.
While it is used alone, the drug can not significantly reduce the pre-menopausal estrogen levels in the blood of women,when it is combined with goserelin (gonadotropin-releasing hormone agonist), its inhibitory effect of estrogen in premenopausal women is greater than goserelin used alone. Formestane has no cross-resistance with other aromatase inhibitors , it has no side effects of aminoglutethimide. After oral administration,it is rapidly absorbed by gastrointestinal, its peak plasma concentration time is 1 to 1.5 hours, but the peak concentration of individual is of great difference; after intramuscular injection,it can be accumulated at the injection site and be slowly absorbed. It performs Biphasic elimination process, the initial elimination half-life is 2 to 4 days, the terminal elimination half-life is 5 to 10 days. It is mainly metabolized in the liver after oral administration, in the form of glycosides acid metabolites excreted in the urine.
The above information is edited by the chemicalbook of Tian Ye.
Chemical Properties
Crystallized from aqueous methanol, m.p. 199~202 ℃; crystallized from ethyl acetate, m.p. 203.5~206 ℃. UV absorption maximum (99.5% ethanol): 278nm (ε11030). [α] D20 + 181 ° (C = 7.7, chloroform).
Uses
aromatase inhibitors ,it is used for progressive breast cancer.
male hormones, assimilating protein class.
production method
Androst-4-ene-3,17-dione (Ⅰ) (1.432g, 5mmo1) is dissolved in 50ml tert-butanol ,add 38mg (0.15mmo1) osmium tetroxide in 2ml t-butanol solution at room temperature, and then 5ml 35% hydrogen peroxide is added, followed by stirring at room temperature for 3 days. After dilution of 100ml brine, and extract with dichloromethane (2 × 100m1). The extract is washed with 100ml brine, , 50ml 10% sodium bisulfite solution, 50ml 10% sodium carbonate solution and 100ml brine, dry over anhydrous sodium sulfate, and concentrate. The residue (1.824g) of the compound (Ⅱ), is dissolved in methanol (10ml),add potassium hydroxide (393mg, 7mmo1) in 3ml methanol . Plus Albert, stirring 10min at 55 ℃. Add 0.3ml of acetic acid and 100ml of brine, and extract with dichloromethane (2 × 100ml). Complex solution, combine, wash with 100ml brine, dry with anhydrous sodium sulfate, and concentrate. The residue (1.727g) by column chromatography on silica gel, wash with hexane-ethyl acetate (7: 3) to give 715mg formestane, yield 47%, mp 200~202 ℃ (acetone).
Description
Formestane is a potent aromatase inhibitor launched in the UK as a second-line endocrine treatment for breast cancer. As a synthetic derivative of androstanedione, the natural substrate for the biosynthesis of estrogen by the enzyme aromatase, formastane selectively inhibits aromatase and binds to its steroid receptor site to cause a rapid and sustained fall in circulating estrogen level and, therefore, inhibits tumor growth. In patients with existing bulky primary tumors, formestane effectively reduces the size of the tumors. Formastane has apparent tolerability advantages and less side effects than other agents such as aminoglutethimide.
Description
4-hydroxy Androstenedione (4-HAD) is a steroidal inhibitor of aromatase (also known as cytochrome P450 19A1; Ki = 27 nM). As aromatase catalyzes the conversion of androgens to estrogens, aromatase inhibitors, including 4-HAD, are used against hormone-sensitive breast cancer in menopausal women. They are also abused in combination with anabolic steroids in racehorses and athletes. This product is intended for forensic and research applications.
Chemical Properties
Needles
Originator
Ciba-Geigy (Switzerland)
History
Formestane is a second-generation steroidal aromatase inhibitor, and the first one to reach clinical use during the early 1990s[1].
Uses
An antitumor drug. An aromatase inhibitor.
Uses
antineoplastic, aromatase inhibitor
Definition
ChEBI: A 17-oxo steroid that is androst-4-ene-3,17-dione in which the hydrogen at position 4 is replaced by a hydroxy group. Formestane was the first selective, type I steroidal aromatase inhibitor, suppressing oestrogen production from anabolic steroids or proho mones. It was formerly used in the treatment of oestrogen-receptor positive breast cancer in post-meopausal women. As it has poor oral bioavailability, it had to be administered by (fortnightly) intramuscular injection. It fell out of use with the subseque t development of cheaper, orally active aromatase inhibitors. Formestane is listed by the World Anti-Doping Agency as a substance prohibited from use by athletes.
brand name
Lentaron
Mechanism of action
Aromatase inhibitors such as formestane and letrozole reduce plasma estradiol levels by inhibiting the conversion of testosterone to estrogen. This compound was first described as a competitive inhibitor, but subsequent evidence proved that its binding to aromatase was irreversible. Hence, it is a suicide inhibitor. In the dog, but not rodents, this results in Leydig cell hypertrophy and hyperplasia. This is thought to be due to the differential sensitivity of the pituitary feedback mechanism to estrogens and androgens in the different species[1-2].
References
[1] Avenda?o C, et al. Anticancer Drugs That Inhibit Hormone Action. Medicinal Chemistry of Anticancer Drugs, 2008; 53-91.0
[2] Berczi I, et al. Biologically Controlled Mutations are Right for Evolution. Insights to Neuroimmune Biology, 2016; 217-241.
Formestane Preparation Products And Raw materials
Raw materials
1of3
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12446 | 58 |
| Hong Kong Excellence Biotechnology Co., Ltd. | ada@sh-teruiop.com | China | 882 | 58 | |
| Nantong Guangyuan Chemicl Co,Ltd | +undefined17712220823 | admin@guyunchem.com | China | 616 | 58 |
| Sigma Audley | +86-15937194204 +86-18126314766 | nova@sh-teruiop.com | China | 491 | 58 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9357 | 55 |
| Rixing Chemical CO.,LTD | +86-13237129059 -13237129059 | sales@rixingchemi.com | CHINA | 229 | 55 |
| Shanxi Naipu Import and Export Co.,Ltd | +86-13734021967 +8613734021967 | kaia@neputrading.com | China | 1011 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29888 | 58 |
| .GZ HONESTCHEM CO.,LTD | +86-15013270415 | honestchemical@foxmail.com | China | 246 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8820 | 58 |
View Lastest Price from Formestane manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-09-19 | Formestane
566-48-3
|
US $100.00 / kg | 1kg | 99.9% | 20tons | Hong Kong Excellence Biotechnology Co., Ltd. | |
 |
2024-04-24 | Formestane
566-48-3
|
US $15.00 / kg | 1kg | 99.912% | 10ton | Ouhuang Engineering Materials (Hubei) Co., Ltd | |
 |
2024-04-19 | Formestane
566-48-3
|
US $3.00-1.00 / kg | 1kg | 99.9% | 10 tons | Shanghai Aosiris new Material Technology Co., LTD |
-

- Formestane
566-48-3
- US $100.00 / kg
- 99.9%
- Hong Kong Excellence Biotechnology Co., Ltd.
-

- Formestane
566-48-3
- US $15.00 / kg
- 99.912%
- Ouhuang Engineering Materials (Hubei) Co., Ltd
-

- Formestane
566-48-3
- US $3.00-1.00 / kg
- 99.9%
- Shanghai Aosiris new Material Technology Co., LTD