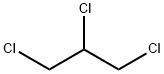
1,2,3-Trichloropropane
- CAS No.
- 96-18-4
- Chemical Name:
- 1,2,3-Trichloropropane
- Synonyms
- TRICHLOROHYDRIN;TRICHLOROPROPANE;1,2,3-Trichlorpropan;1,2,3-Trichloropropa;NCI-C60220;oropropane;1,2,3-TrichL;allyltrichloride;Allyl trichloride;3-trichloropropane
- CBNumber:
- CB6854719
- Molecular Formula:
- C3H5Cl3
- Molecular Weight:
- 147.43
- MDL Number:
- MFCD00000946
- MOL File:
- 96-18-4.mol
| Melting point | ?14 °C (lit.) |
|---|---|
| Boiling point | 156 °C (lit.) |
| Density | 1.387 g/mL at 25 °C (lit.) |
| vapor pressure | 3.1 at 25 °C (Banerjee et al., 1990) |
| refractive index |
n |
| Flash point | 180 °F |
| storage temp. | 2-8°C |
| solubility | Soluble in alcohol and ether (Weast, 1986); miscible with propyl chloride, carbon tetrachloride, and chloroform. |
| color | Colorless to Almost colorless |
| Water Solubility | 2 g/L (25 ºC) |
| BRN | 1732068 |
| Henry's Law Constant | 34.5 at 25.0 °C (mole fraction ratio-GC, Leighton and Calo, 1981) |
| Exposure limits | TLV-TWA 10 ppm (~60 mg/m3) (ACGIH), 50 ppm (MSHA, OSHA, and NIOSH); IDLH 1000 ppm (NIOSH). |
| Dielectric constant | 2.3(29℃) |
| LogP | 2.63 at 25℃ |
| CAS DataBase Reference | 96-18-4(CAS DataBase Reference) |
| Indirect Additives used in Food Contact Substances | 1,2,3-TRICHLOROPROPANE |
| FDA 21 CFR | 177.1650 |
| EWG's Food Scores | 5 |
| FDA UNII | 3MJ7QCK0Z0 |
| Proposition 65 List | 1,2,3-Trichloropropane |
| NIST Chemistry Reference | Propane, 1,2,3-trichloro-(96-18-4) |
| IARC | 2A (Vol. 63) 1995 |
| EPA Substance Registry System | 1,2,3-Trichloropropane (96-18-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS06,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301+H311+H331-H319-H341-H350-H360F-H372-H412 | |||||||||
| Precautionary statements | P273-P280-P301+P310-P302+P352+P312-P304+P340+P311-P305+P351+P338 | |||||||||
| Hazard Codes | T,F,N | |||||||||
| Risk Statements | 45-60-20/21/22-39/23/24/25-23/24/25-11-38-68/20/21/22-48/23-48/21/22-36-24/25-20 | |||||||||
| Safety Statements | 53-45-36/37-37-36-61-26-16-7 | |||||||||
| RIDADR | UN 2810 6.1/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | TZ9275000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29031980 | |||||||||
| Toxicity | Acute oral LD50 for rats 320 mg/kg (quoted, RTECS, 1985). | |||||||||
| IDLA | 100 ppm | |||||||||
| NFPA 704 |
|
1,2,3-Trichloropropane price More Price(12)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 48355 | 1,2,3-Trichloropropane solution certified reference material, 200?μg/mL in methanol | 96-18-4 | 1mL | $31.9 | 2024-03-01 | Buy |
| Sigma-Aldrich | 47794 | 1,2,3-Trichloropropane analytical standard | 96-18-4 | 1000mg | $36.9 | 2024-03-01 | Buy |
| TCI Chemical | T0395 | 1,2,3-Trichloropropane >98.0%(GC) | 96-18-4 | 25g | $16 | 2024-03-01 | Buy |
| Alfa Aesar | L04312 | 1,2,3-Trichloropropane, 98+% | 96-18-4 | 250g | $39.1 | 2024-03-01 | Buy |
| Alfa Aesar | L04312 | 1,2,3-Trichloropropane, 98+% | 96-18-4 | 1000g | $98.9 | 2024-03-01 | Buy |
1,2,3-Trichloropropane Chemical Properties,Uses,Production
Description
1,2,3-Trichloropropane is a chemical compound existing as an intermediate in certain pesticides. It is used as the intermediate for the manufacturing of chemicals such as epoxy resins, polysulfone liquid polymers, the synthesis of hexafluoropropylene, and as a cross-linking agent in the synthesis of polysulfides. It has also been used as the solvent for paint and varnish removal, a cleaning and degreasing agent, and a maintenance solvent. It has also been used as a soil fumigant. In addition, it is also a “branching” or curing agent in polysulfide polymers, which is used as the sealants for aircraft fuel tanks and bodies. However, it should be noted that it is a persistent groundwater pollutant and a potential human carcinogen.
Chemical Properties
1,2,3-Trichloropropane (hereinafter referred to as TCP) is a chlorinated hydrocarbon that was historically used as an industrial solvent and a degreasing agent. Currently, however, TCP is utilized as an intermediate in the production of polymer cross-linking agents, pesticides, and glycerol. In its pure form, TCP is a colorless to yellow liquid with limited solubility in water, a strong chloroform-like odor, moderate volatility, and high flammability.
Source
Produced in large quantities as an epichlorohydrin production byproduct, TCP is a synthetic compound that does not occur naturally in the environment. In the agrochemical industry, TCP is formed via the manufacture of dichloropropene-derived nematicides (pesticides used to kill parasitic nematodes), and it is also present as an impurity in these soil fumigants. As a result, application of these products has produced significant atmosphere, soil, and groundwater contamination, which in turn can induce various health problems in wildlife and humans. The toxicological effects of TCP depend on dose and duration, but can range from kidney and liver damage to tumors and cancers.
health standards
The EPA has established drinking water health advisories for 1,2,3-Trichloropropane, which are drinking water specific risk level concentrations for cancer (10-4 cancer risk) and concentrations of drinking water contaminants at which noncancer adverse health effects are not anticipated to occur over specific exposure durations. EPA established a 1-day health advisory of 0.6 milligrams per liter (mg/L) and a 10-day health advisory of 0.6 mg/L for TCP in drinking water for a 10 kilogram (kg) child (EPA 2012a).
Reference
- Samin, G, and D. B. Janssen. "Transformation and biodegradation of 1,2,3-trichloropropane (TCP). " Environmental Science & Pollution Research 19.8(2012): 3067-3078.
- Liu, P., et al. "Successful therapy with hemoperfusion and plasma exchange in acute 1,2,3-trichloropropane poisoning." Human & Experimental Toxicology 31.5(2012): 523-527.
Description
1,2,3-Trichloropropane is a synthetic chemical that is also known as allyl trichloride, glycerol trichlorohydrin, and trichlorohydrin. It is a colourless, heavy liquid with a sweet but strong chloroform-like odour and is combustible. It is slightly soluble in water but soluble in chloroform, diethyl ether, and ethanol. On contact with heat/ fire, It releases off irritating or toxic fumes (or gases). It evaporates very quickly and small amounts dissolve in water. It is mainly used to make other chemicals. 1,2,3-Trichloropropane was used in the past mainly as a solvent and extractive agent, including as a paint and varnish remover and as a cleaning and degreasing agent. It is now used mainly as a chemical intermediate, for example, in the production of polysulphone liquid polymers, dichloropropene and hexafluoropropylene and as a cross-linking agent in the synthesis of polysulphides.
Chemical Properties
1,2,3-Trichloropropane is a colorless liquid with a strong acid odor. slightly soluble in water; dissolves oils, fats, waxes, chlorinated rubber, and numerous resins. autoign temp 580F (304°C). Combustible.
Physical properties
Clear, colorless liquid with a strong, chloroform-like odor
Uses
Intermediate in the manufacture of pesticides and polysulfide rubbers; formerly used as a solvent and extractive agent.
Uses
Paint and varnish remover, solvent, degreasing agent.
Uses
1,2,3-Trichloropropane is used as a solventand as an intermediate in organic synthesis.
Definition
ChEBI: 1,2,3-Trichloropropane is an organochlorine compound.
General Description
Colorless liquid with a strong acid odor. Denser than water and slightly soluble in water. Hence sinks in water.
Reactivity Profile
1,2,3-Trichloropropane is sensitive to prolonged exposure to light. Sensitive to heat. May react with active metals, strong caustics and oxidizing agents. Attacks some plastics, rubber and some coatings .
Health Hazard
Inhalation of vapor causes anesthesia, dizziness, and nausea. Vapor is highly irritating by inhalation routes and moderately irritating by dermal routes. Exposure of eyes to vapor may result in slight, transient injury to the cornea.
Health Hazard
Inhalation of its vapors can produce depres sion of the central nervous system, which canprogress to narcosis and convulsion as theconcentration increases. A 30-minute expo sure to a 5000-ppm concentration causedconvulsions in rats. Acute as well as chronicexposure to high concentrations can causeliver damage. 1,2,3-Trichloropropane is moretoxic than its 1,1,1-isomer. Acute oral tox icity is moderate, with LD50 values rang ing between 300 and 550 mg/kg in differentspecies of experimental animals. The liquidis a strong irritant to the eyes.
Fire Hazard
Combustible liquid; flash point (closed cup) 73°C (164°F), (open cup) 82°C (180°F). Vapors of 1,2,3-trichloropropane form explo sive mixtures with air, with LEL and UEL values of 3.2% and 12.6% by volume in air, respectively. The compound reacts vigorously with alkali metals, powdered magnesium, or aluminum; caustic alkalies; and oxidizers.
Flammability and Explosibility
Non flammable
Safety Profile
Confirmed carcinogen. Poison by ingestion. Moderately toxic by inhalation and skin contact. Experimental reproductive effects. A skin and severe eye irritant. Mutation data reported. Moderately flammable by heat, flames (sparks), or powerful oxidizers. See also ALLYL COMPOUNDS and CHLORINATED HYDROCARBONS, ALIPHATIC. When heated to decomposition it yields hghly toxic Cl-. To fight fre, use water (as a blanket), spray, mist, dry chemical.
Potential Exposure
Trichloropropane dissolves oils, fats, waxes, chlorinated rubber; and numerous resins; it is used as a paint and varnish remover; a solvent; and a degreasing agent.
Carcinogenicity
1,2,3-Trichloropropane is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals.
Environmental Fate
Chemical/Physical. The hydrolysis rate constant for 1,2,3-trichloropropane at pH 7 and 25 °C
was determined to be 1.8 x 10-6/h, resulting in a half-life of 43.9 yr (Ellington et al., 1988). The
hydrolysis half-lives decrease at varying pHs and temperature. At 87 °C, the hydrolysis half-lives
at pH values of 3.07, 7.12, and 9.71 were 21.1, 11.6, and 0.03 d, respectively (Ellington et al.,
1986). By analogy to 1,2-dibromo-2-chloropropane, the following hydrolysis products would be
formed: 2,3-dichloro-1-propanol, 2,3-dichloropropene, epichlorohydrin, 1-chloro-2,3-
dihydroxypropane, glycerol, 1-hydroxy-2,3-propylene oxide, 2-chloro-3-hydroxypropene, and
HCl (Kollig, 1993).
The volatilization half-life of 1,2,3-trichloropropane (1 mg/L) from water at 25 °C using a
shallow-pitch propeller stirrer at 200 rpm at an average depth of 6.5 cm was 56.1 min (Dilling,
1977).
Shipping
UN2810 Toxic liquids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.
Toxicity evaluation
It is currently believed that TCP itself has very low activity, while its metabolites mainly cause toxicity, carcinogenicity, and other effects. The liver can metabolize TCP by cytochrome P450 enzymes to give reactive intermediates, which bind to glutathione or other protein for excretion. The reactive intermediates can bind to DNA, proteins, and other molecules and cause hepatocellular damage, gene mutation, and organ dysfunction. Activation of the molecule may occur by reaction with glutathione. The metabolites (e.g., dichloroacetone) accumulated through glutathione depletion and consequently covalent binding to DNA and other macromolecules such as microsomal proteins.
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Violent decomposition with chemically active metals; strong bases. Keep away from chlorinated rubber, resins and waxes; and sunlight.
Waste Disposal
Incineration, preferably after mixing with another combustible fuel. Care must be exercised to assure complete combustion to prevent the formation of phosgene. An acid scrubber is necessary to remove the halo acids produced.
1,2,3-Trichloropropane Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 15928 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | sales@amoychem.com | China | 6387 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49390 | 58 |
| career henan chemical co | +86-0371-86658258 15093356674; | factory@coreychem.com | China | 29826 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | sales@tnjchem.com | China | 34572 | 58 |
| AFINE CHEMICALS LIMITED | 0571-85134551 | info@afinechem.com | CHINA | 15377 | 58 |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | 1026@dideu.com | China | 9116 | 58 |
View Lastest Price from 1,2,3-Trichloropropane manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-07-27 | 1,2,3-Trichloropropane
96-18-4
|
US $1.50 / g | 1g | 99.0% Min | 100 Tons | Shaanxi Didu New Materials Co. Ltd | |
 |
2021-07-02 | 1,2,3-Trichloropropane 99%
96-18-4
|
US $15.00-10.00 / KG | 1KG | 99%+ HPLC | Monthly supply of 1 ton | Zhuozhou Wenxi import and Export Co., Ltd | |
 |
2020-01-08 | 1,2,3-Trichloropropane
96-18-4
|
US $2.00 / KG | 1KG | 98% | 100g , 1kg, 5kg , 50kg | Career Henan Chemical Co |
-

- 1,2,3-Trichloropropane
96-18-4
- US $1.50 / g
- 99.0% Min
- Shaanxi Didu New Materials Co. Ltd
-

- 1,2,3-Trichloropropane 99%
96-18-4
- US $15.00-10.00 / KG
- 99%+ HPLC
- Zhuozhou Wenxi import and Export Co., Ltd
-

- 1,2,3-Trichloropropane
96-18-4
- US $2.00 / KG
- 98%
- Career Henan Chemical Co