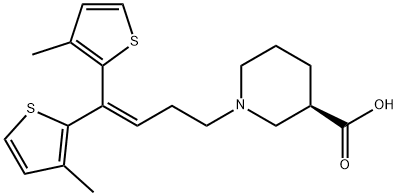
Tiagabine
- CAS No.
- 115103-54-3
- Chemical Name:
- Tiagabine
- Synonyms
- TGB;no328;Tiagabin;TIGABINE;TIAGABINE;NO-05-0328;nnc-05-0328;Tiagabine D4;NNC-05-0328:A-70569;Gabitril / NNC 05-328
- CBNumber:
- CB9761920
- Molecular Formula:
- C20H25NO2S2
- Molecular Weight:
- 375.55
- MDL Number:
- MFCD00865317
- MOL File:
- 115103-54-3.mol
- MSDS File:
- SDS
| Melting point | 192oC dec. |
|---|---|
| Boiling point | 568.0±50.0 °C(Predicted) |
| Density | 1.208±0.06 g/cm3(Predicted) |
| storage temp. | under inert gas (nitrogen or Argon) at 2-8°C |
| pka | 3.86±0.20(Predicted) |
| Water Solubility | ≥ 13.5mg/mL in Water |
| CAS DataBase Reference | 115103-54-3(CAS DataBase Reference) |
| FDA UNII | Z80I64HMNP |
| ATC code | N03AG06 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H315-H319-H335 |
| Precautionary statements | P261-P305+P351+P338 |
| Hazard Codes | Xi |
| Risk Statements | 36/37/38 |
| Safety Statements | 26-37/39 |
Tiagabine price
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Medical Isotopes, Inc. | D34367 | Tiagabine-d4HCl | 115103-54-3 | 1mg | $665 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | API0009125 | TIAGABINE 97.00% | 115103-54-3 | 1G | $675.15 | 2021-12-16 | Buy |
| AvaChem | 2070B | Tiagabine | 115103-54-3 | 10mg | $45 | 2021-12-16 | Buy |
| AvaChem | 2070B | Tiagabine | 115103-54-3 | 100mg | $105 | 2021-12-16 | Buy |
| Crysdot | CD31004467 | Tiagabine 98+% | 115103-54-3 | 50mg | $119 | 2021-12-16 | Buy |
Tiagabine Chemical Properties,Uses,Production
Description
Tiagabine is a second- generation antiepileptic drug (AED) known under the proprietary brand name of Gabitril® (Teva, Petah Tikva, Israel) in the UK and USA.
Generic formulation
MHRA/ CHM advice to minimize risk when switching patients with epilepsy between different manufacturers’ products (including generic products):
- It is usually unnecessary to ensure that patients are maintained on a specific manufacturer’s product unless there are specific concerns, such as patient anxiety and risk of confusion/ dosing error.
Indications
Epilepsy: adjunctive therapy for focal seizures with or without secondary generalization that are not satisfactorily controlled by other AEDs.
Recommendations summarized from NICE (2012)
- Seizure types: on referral to tertiary care (focal seizures), contraindicated (generalized tonic- clonic seizures, tonic/ atonic seizures, absence seizures, myoclonic seizures).
- Epilepsy types: on referral to tertiary care (benign epilepsy with centrotemporal spikes, panayiotopoulos syndrome, late- onset childhood occipital epilepsy), contraindicated (absence syndromes, idiopathic generalized epilepsy, juvenile myoclonic epilepsy, Dravet syndrome, Lennox– Gastaut syndrome).
Dose titration
- Epilepsy— adjunctive therapy (with enzyme- inducing AEDs): 5–10 mg daily divided into 1 or 2 doses for 7 days, then increased by 5–10 mg daily every 7 days; usual maintenance 30– 45 mg daily divided into 2 or 3 doses.
- Epilepsy— adjunctive therapy (without enzyme- inducing AEDs): 5–10 mg daily divided into 1 or 2 doses for 7 days, then increased by 5–10 mg daily every 7 days; usual maintenance 30– 45 mg daily divided into 2–3 doses.
Plasma levels monitoring
The inter- individual variation in liver metabolism makes Tiagabine a strong candidate for therapeutic drug monitoring. A broad reference range of 20– 200 ng/ mL has been proposed, however, the relatively short half- life of Tiagabine under most conditions means that care must be taken in drawing blood for therapeutic drug monitoring. The high binding to serum proteins further suggests that measurement of free drug concentrations may be useful. However, there has been little investigation of the relationship between serum/ plasma concentrations and therapeutic efficacy.
Cautions
- Patients with acute porphyrias.
- Patients with absence, myoclonic, tonic and atonic seizures (risk of exacerbation).
Adverse effects
Tiagabine can be associated with adverse effects at the level the nervous system and other systems.
Interactions
With AEDs
- AEDs that induce hepatic enzymes (such as carbamazepine, phenytoin, phenobarbital, and primidone) enhance the metabolism of tiagabine: the plasma concentration of tiagabine may be reduced by a factor .5– 3 by concomitant use of these AEDs.
- Tiagabine reduces the plasma concentration of valproate by about 0% (this is not considered clinically important and does not warrant a dose modification).
- Cimetidine increases the bioavailability of tiagabine by about 5% (this is not considered clinically important and does not warrant a dose modification).
- The combination of tiagabine with St John Wort (Hypericum perforatum) may lead to lower exposure and loss of efficacy of tiagabine, due to the potent induction of CYP3A4 by St John Wort, resulting in increased tiagabine metabolism. Therefore, the combination of tiagabine with St John’s Wort is contraindicated.
There are no known specific interactions between alcohol and tiagabine and there are no specific foods that must be excluded from diet when taking tiagabine. Administration with food results in a decreased rate and not extent of absorption
Special populations
Hepatic impairment
- Reduce dose, prolong the dose interval, or both, in mild to moderate impairment.
- Avoid in severe impairment.
Renal impairment
Renal insufficiency does not affect the pharmacokinetics of Tiagabine, therefore its dosage does not need to be modified.
Pregnancy
- Clinical experience of the use of tiagabine in pregnant women is limited and no information on tiagabine during breastfeeding is available. Therefore, as a precautionary measure, it is preferable not to use tiagabine during pregnancy or breast- feeding unless the potential benefits of treatment outweigh the potential risks.
- In case of tiagabine treatment during pregnancy, the dose should be monitored carefully and adjustments made on a clinical basis.
Behavioural and cognitive effects in patients with epilepsy
Treatment with tiagabine has often been associated with depression and irritability. Results from randomized double- blind, controlled trials with tiagabine as adjunctive treatment have confirmed the incidence of psychiatric problems, which can be mild- to- moderate in severity and can be reported more frequently by patients with a personal history of affective disorders, or in case of rapid initial titration. Tiagabine is characterized by a good profile in terms of cognitive adverse effects, with mild effects on concentration and memory, which can be minimized by slow initial titration.
Psychiatric use
Tiagabine has no approved indications in psychiatry and there is no conclusive evidence for its efficacy in the treatment of any behavioural problems.
Description
Gabitril was launched in Denmark for use as an add-on therapy in patients refractory to other epilepsy therapies. The compound can be synthesized in five steps beginning with a bis-thiophenyl ketone derivative to produce the (R)-(-)- enantiomer. Its anti-epileptic activity resides in its potent and selective inhibition of GABA synaptosomal uptake. Tiagabine is selective for the GAT-1 GABA transporter in neurons and glia thus enhancing inhibitory GABAergic transmission. Because it has practically no effect on other uptake or receptor systems, it has a reduced potential for neurological side-effects. In particular, it does not have the benzodiazepine-like sedative effects. It is able to cross the blood brain barrier and is considered the most potent GABA uptake inhibitor known.
Chemical Properties
White to Off-White Crystalline Solid
Originator
Novo Nordisk (Denmark)
Uses
A GABA uptake inhibitor
Definition
ChEBI: A piperidinemonocarboxylic acid that is (R)-nipecotic acid in which the hydrogen attached to the nitrogen has been replaced by a 1,1-bis(3-methyl-2-thienyl)but-1-en-4-yl group. A GABA reuptake inhibitor, it is used (generally as the hydroc loride salt) for the treatment of epilepsy.
Manufacturing Process
A solution of 34 ml of n-butyl lithium in 30 ml of anhydrous ether was cooled
to -65°C under nitrogen and 5.3 ml of 3-methyl-2-bromothiophene in 10 ml
anhydrous ether was added dropwise over a period of 10 min. The reaction
mixture was stirred at -65°C for 1 h and 2.7 ml of ethyl 4-bromo-butyrate in
10 ml of anhydrous ether was added slowly. The reaction was stirred for 4 h
while the temperature raised to -20°C, 20 ml water was added, and the
mixture was stirred for 5 min after which the aqueous layer was removed. The
ether layer was washed with 20 ml of water, and the combined aqueous
phases were extracted with 50 ml of ether. The combined organic phases were
dried over anhydrous sodium sulfate, which after evaporation yielded 9 g of 1-
bromo-4,4-bis(3-methylthien-2-yl)but-3-ene as an oil.
This compound was without further purification used for coupling with ethyl
nipecotate.
A suspension of 5.0 g of 1-bromo-4,4-bis(3-methylthien-2-yl)but-3-ene, 3.4 g
of nipecotic acid ethyl ester and 3.3 g of potassium carbonate in 150 ml of dry
acetone was kept under reflux for 15 h. The reaction mixture was evaporated
and, after addition of 30 ml of water, the resulting solution was extracted
twice with 50 ml of ethyl acetate. The ethyl acetate extracts were dried and
evaporated leaving 7.3 g of an oil. By column chromatography on silica gel
using methanol as eluent, N-(4,4-bis(3-methylthien-2-yl)but-3-enyl)nipecotic
acid ethyl ester was isolated.
5.3 g of N-(4,4-bis(3-methylthien-2-yl)but-3-enyl)nipecotic acid ethyl ester
was dissolved in 100 ml of ethanol and 200 ml of an 8 N sodium hydroxide
solution was added. The mixture was heated at reflux for 1 h, cooled and
acidified by adding 10% hydrochloric acid. The resulting solution was
evaporated and 100 ml of water was added to the residue. The resulting acid
solution was extracted with ethyl acetate and the dried extract was
evaporated to give (R)-N-(4,4-bis(3-methylthien-2-yl)but-3-enyl)nipecotic acid
hydrochloride, melting point 187°-189°C.
brand name
Gabitril
Therapeutic Function
Antiepileptic
Biological Functions
Tiagabine (Gabitril) blocks the reuptake of GABA into neurons and glia, thereby resulting in higher levels of GABA in the synaptic cleft. The ability to increase GABA concentrations is presumed to be involved in the effectiveness of tiagabine in the treatment of seizure disorders. It is primarily used in the treatment of partial complex seizures.Adverse effects of tiagabine administration include dizziness, somnolence, nervousness, nausea, and confusion.
General Description
A glance at tiagabine’s structure suggests anuptake inhibitor. Reportedly, it blocks GABA reuptake asa major mode of its anticonvulsant activity. Its use isagainst partial seizures. Inhibitors of GABA transporter-1(GAT-1 inhibitors) increase extracellular GABA concentrationin the hippocampus, striatum, and cortex, therebyprolonging the inhibitory action of GABA released synaptically.Nipecotic acid is a potent inhibitor of GABA reuptakeinto synaptosomal membranes, neurons, and glialcells. However, nipecotic acid fails to cross the blood-brainbarrier following systemic administration because of itshigh degree of ionization. Tiagabine, marketed as thesingle R(-)-enantiomer, a potent GAT-1 inhibitor structurallyrelated to nipecotic acid, has an improved ability tocross the blood-brain barrier, and it has recently receivedFood and Drug Administration (FDA) approval as anAED.It is well absorbed and readily metabolized byCYP3A4 to an inactive metabolite, 5-oxo-tiagabine (oxidationof the thiophen ring) or eliminated as glucuronide ofthe parent molecule.
Over 90% of tiagabine is metabolized by CYP3A4isozymes.The primary site of metabolic attack is the oxidationof the thiophen rings leading to 5-oxo-tiagabine thatlacks anticonvulsant activity and the glucuronidation via thecarboxylic function. Thus, the plasma concentrations oftiagabine would be greatly effected by any compound thatinduces or inhibits CYP3A4.
Mechanism of action
Tiagabine is a nipecotic acid derivative with an improved ability to cross the blood-brain barrier. It was rationally designed to be a GABA uptake inhibitor based on the fact that nipecotic acid (piperidine-3-carboxylic acid) inhibits GABA uptake by glial cells. Tiagabine binds to the GABA transporter GAT1, blocking the uptake of GABA into both neurons and glia, thus enhancing GABA-mediated inhibition. Tiagabine is presently approved for adjunct use in patients with epilepsy who are older than 12 years and have partial seizures not controlled by first-line drugs.
Pharmacokinetics
Tiagabine is well absorbed, with an oral bioavailability of 90 to 95%. It displays linear pharmacokinetics, with a plasma half-life of 5 to 8 hours, necessitating a multiple daily dosing regimen. It also is highly protein bound (96%). The major pathway of metabolism for tiagabine is oxidation by CYP3A4, followed by glucuronidation. Its pharmacokinetics are altered by the coadministration of enzyme-inducing AEDs, even though tiagabine does not appear to induce or inhibit hepatic microsomal metabolizing enzymes.
Clinical Use
Antiepileptic
Side effects
Side effects are more common with tiagabine than with other adjunct drugs and most often involve the CNS. They include
somnolence, headache, dizziness, tremor, abnormal thinking, depression, and psychosis. Furthermore, recent reports have
implicated tiagabine in the development of nonconvulsive status epilepticus. There is an increased risk of seizure in
patients being treated for off-label psychiatric indications. Tiagabine may interfere with visual color perception.
Tiagabine does not affect the hepatic metabolism of other AEDs, but its half-life is decreased by enzyme-inducing AEDs, such
as CBZ, phenytoin, and barbiturates. Other CYP3A4-inducing drugs may act similarly. Valproate decreases the protein binding
of tiagabine. increasing its plasma concentration in these patients.
Hepatic disease causes decreased clearance of tiagabine, and a dose reduction may be required. Renal disease does not
affect elimination.
Drug interactions
Potentially hazardous interactions with other drugs
Antidepressants: antagonism of anticonvulsant effect with SSRIs, tricyclics and MAOIs (convulsive threshold lowered); avoid with St John’s wort.
Antiepileptics: concentration reduced by phenytoin, carbamazepine and phenobarbital.
Antimalarials: mefloquine antagonises anticonvulsant.
Antipsychotics: anticonvulsant effect antagonised.
Orlistat: possibly increased risk of convulsions.
Metabolism
Tiagabine has negligible renal clearance. Hepatic metabolism is the principle route for elimination of tiagabine. Less than 2
% of the dose is excreted unchanged in urine and faeces. No active metabolites have been identified.
Tiagabine Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49390 | 58 |
| career henan chemical co | +86-0371-86658258 15093356674; | factory@coreychem.com | China | 29826 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| HANGZHOU CLAP TECHNOLOGY CO.,LTD | 86-571-88216897,88216896 13588875226 | sales@hzclap.com | CHINA | 6313 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418684 +8618949823763 | sales@tnjchem.com | China | 25363 | 58 |
| Hangzhou MolCore BioPharmatech Co.,Ltd. | +86-057181025280; +8617767106207 | sales@molcore.com | China | 49739 | 58 |
| Wuhan Topule Biopharmaceutical Co., Ltd | +8618327326525 | masar@topule.com | China | 8474 | 58 |
| LEAPCHEM CO., LTD. | +86-852-30606658 | market18@leapchem.com | China | 43348 | 58 |
| Shanghai Acmec Biochemical Technology Co., Ltd. | +undefined18621343501 | product@acmec-e.com | China | 33349 | 58 |
| Aladdin Scientific | +1-833-552-7181 | sales@aladdinsci.com | United States | 57511 | 58 |