ナフタレン 化学特性,用途語,生産方法
外観
白色, 結晶又は塊
性質
ナフタレンには独特の匂いがあり、常温で昇華性を示します。ナフタレンは、エーテル、など多くの有機溶剤には溶けますが、水には不溶です。
ナフタレンは2組の等価な水素原子を持っています。α位は1、4、5、8位で、β位は2、3、6、7位です。
性質
ナフタリンは有機化合物の中でも比較的低分子量の化合物であり、水にはほとんど溶けない無極性分子です。しかし、有機溶媒には溶けやすい性質があります。そのためナフタリンは石油エーテル、アルコール、ベンゼン、トルエンなどの有機溶媒に容易に溶けます。
また、ナフタリンは分子内に二重結合や極性基を持たないため、分子間力 (ファンデルワールス力) で結合しているという特徴があります。従って、分子量が大きくなると分子間力も増大し、ナフタレン、アントラセン、フェナントレン、ピレンなどの高分子多環芳香族炭化水素は、ナフタリンと比べて分子間力が強く、不揮発性・高融点・難溶性などの性質を持ちます。また、ナフタリンは空気中の酸素と反応して酸化され、光や熱によっても容易に酸化されます。
このため、ナフタリンを含む製品は、適切な密閉容器に保管する必要があります。水酸化ナトリウムや硫酸などの強い酸化剤に対して不安定であり、爆発的な反応を起こすことがあるため、これらの物質と混合させないように注意が必要です。
反応性
ナフタレンもベンゼンと同じように、芳香族求電子置換反応を受けます。ただし、ナフタレンはベンゼンと比べて穏和な条件で反応する場合が多いです。
したがって、ベンゼン上で起きる反応は、ナフタレンでも起こります。位置選択性は反応により違いますが、速度論的に有利なのはα位の置換で、熱力学的に有利なのはβ位の置換です。
溶解性
水に難溶 (3mg/100ml), 各種有機溶剤に可溶。エタノールにやや溶けやすく、水にほとんど溶けない。
解説
化学式はC1(/0)H8。二環式の縮合環をもつ芳香族炭化水素。製品の場合はナフタリンと呼ぶ。特有臭のある白色の結晶。融点80.5℃,沸点217.9℃。水に不溶,有機溶媒に可溶。昇華性。塩素化,スルホン化,ニトロ化などの反応を受ける。コールタールの中油およびナフタレン油留分などから得られる。染料中間体,防虫剤,爆薬,界面活性剤などの原料,また酸化して得られる無水フタル酸はポリエステル系の繊維,合成樹脂の原料として重要。
解説
C10H8(128.17).石炭タールのナフタレン留分(沸点200~250 ℃)中に多量に存在する炭化水素.また,石油原油および改質油中にも微量存在する. "コールタールのナフタレン油から酸性および塩基性物質を抽出して除き,精密蒸留するか,あるいは冷却して結晶を析出させ,遠心分離によって油分を除けば,粗ナフタレンが得られる.合成するには,ベンゼンとアセチレンの混合物を赤熱管中に通じるなどの方法がある.強いコールタール臭をもつ白色の結晶.融点80.3 ℃,沸点218.0 ℃.d204"1.162.n25D"1.5898.λmax 220,275,314 nm(log ε 5.05,3.75,2.50,エタノール).昇華性がある.水に不溶,エタノール,エーテル,ベンゼンなど多くの有機溶媒に可溶.ポリニトロベンゼンやピクリン酸と付加物をつくる.ナフタレンの蒸気を赤熱管中に通すと,2,2′-ビナフチルを生じる.また,酸化すれば1,4-ナフトキノンを経てフタル酸が,水素付加すればテトラリンを経てデカリンが合成できる.ベンゼンと同様,多くの親電子試薬と反応して種々の核置換体を生じるが,ベンゼンよりも反応性が高いため,ジ置換体以上のポリ置換体が生じやすい.また,一般に1位のほうが2位よりも置換されやすい.染料中間体原料,殺虫剤に用いられる.[CAS 91-20-3] 森北出版「化学辞典(第2版)
"コールタールのナフタレン油から酸性および塩基性物質を抽出して除き,精密蒸留するか,あるいは冷却して結晶を析出させ,遠心分離によって油分を除けば,粗ナフタレンが得られる.合成するには,ベンゼンとアセチレンの混合物を赤熱管中に通じるなどの方法がある.強いコールタール臭をもつ白色の結晶.融点80.3 ℃,沸点218.0 ℃.d204"1.162.n25D"1.5898.λmax 220,275,314 nm(log ε 5.05,3.75,2.50,エタノール).昇華性がある.水に不溶,エタノール,エーテル,ベンゼンなど多くの有機溶媒に可溶.ポリニトロベンゼンやピクリン酸と付加物をつくる.ナフタレンの蒸気を赤熱管中に通すと,2,2′-ビナフチルを生じる.また,酸化すれば1,4-ナフトキノンを経てフタル酸が,水素付加すればテトラリンを経てデカリンが合成できる.ベンゼンと同様,多くの親電子試薬と反応して種々の核置換体を生じるが,ベンゼンよりも反応性が高いため,ジ置換体以上のポリ置換体が生じやすい.また,一般に1位のほうが2位よりも置換されやすい.染料中間体原料,殺虫剤に用いられる.[CAS 91-20-3] 森北出版「化学辞典(第2版)
用途
染色中間体、殺虫剤、殺菌剤、爆薬、酸化防止剤、溶剤、プラスチック原料、防虫剤、無水フタル酸原料
用途
環境(大気)分析用標準品。
用途
染料中間物、有機顔料
構造
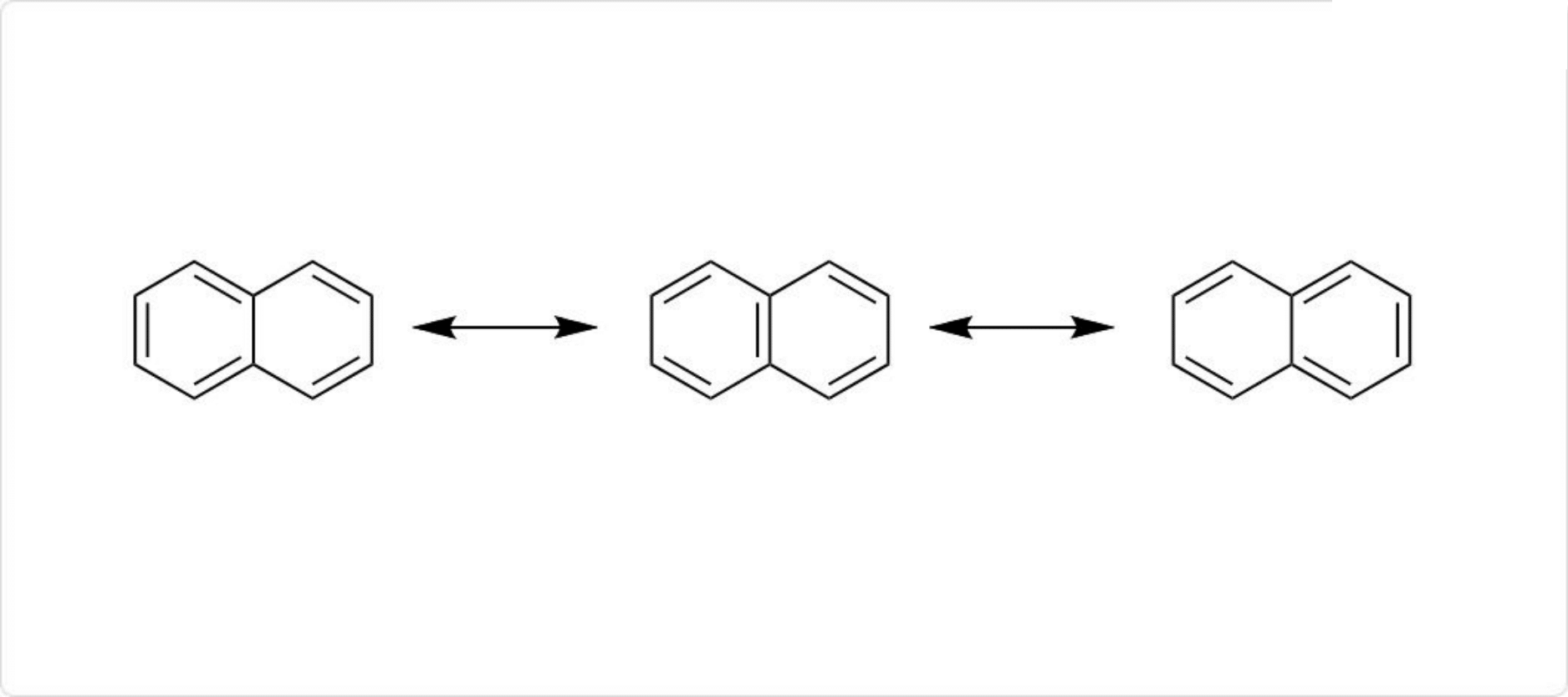
ナフタレンの共鳴構造
ナフタレンは、1辺を2個のベンゼン環が共有している構造を持った多環芳香族炭化水素です。分子式はC10H8、分子量は128.17です。
ナフタレンの炭素-炭素結合は、ベンゼンとは異なり、すべて同じ結合長ではありません。C1-C2、C3-C4、C5-C6、C7-C8間の炭素-炭素結合はおよそ136pmですが、それ以外の炭素-炭素結合の長さはおよそ142 pmです。この違いは3種類の共鳴構造を含めたナフタレン中の結合の原子価結合モデルによって説明できます。
すなわち、C1-C2、C3-C4、C5-C6、C7-C8間の炭素-炭素結合は3種類の共鳴構造のうち2種類が二重結合ですが、それ以外の炭素-炭素結合は共鳴構造のうち1種類のみが二重結合であるためです。
ナフタレンはアセン類 (英: acene) の最も単純な化合物です。ナフタレンの構造異性体には、5員環と7員環で構成される (英: azulene) があります。
構造
ナフタリンの構造は、2つのベンゼン環が共有結合している多環芳香族炭化水素です。ベンゼン環と同様に、ナフタリンの構造は平面的であり、二次元的に描写されます。ナフタレンには、1つの置換基を持つ一置換体があります。この一置換体には2種類の構造異性体が存在し、1,4,5,8位に存在する置換基をα位、2,3,6,7位に存在する置換基をβ位と呼びます。
説明
Naphthalene occurs as transparent prismatic plates also available as white scales, powder
balls, or cakes with a characteristic mothball or strong coal tar and aromatic odour. It is
sparingly soluble in water but soluble in methanol/ethanol and very soluble in ether.
Naphthalene is a commercially important aromatic hydrocarbon. Naphthalene occurs as
a white solid or powder. Naphthalene occurs in coal tar in large quantities and is easily
isolated from this source in pure condition. It volatilises and sublimes at room temperature
above the melting point. The primary use for naphthalene is in the production of
phthalic anhydride, also of carbamate insecticides, surface active agents and resins, as a
dye intermediate, as a synthetic tanning agent, as a moth repellent, and in miscellaneous
organic chemicals. Naphthalene is used in the production of phthalic anhydride; it is also
used in mothballs. Naphthalene is also used in the manufacture of phthalic and anthranilic
acids to make indigo, indanthrene, and triphenyl methane dyes, for synthetic resins,
lubricant, celluloid, lampblack, smokeless powder, and hydronaphthalenes. Naphthalene
is also used in dusting powders, lavatory deodorant discs, wood preservatives, fungicide,
and as an insecticide. It has been used as an intestinal antiseptic and vermicide and in
the treatment of pediculosis and scabies.
化学的特性
Naphthalene is a colorless to brown crystalline solid with a characteristic “moth ball” odor. It evaporates easily and has a strong odor of tar or mothballs. Solubility in water is low (31.7 mg/l at 25 °C), and it is soluble in benzene, alcohol, ether, and acetone (ATSDR, 2005). Shipped as a molten solid.
来歴
In 1819, naphthalene was obtained as white crystals during the pyrolysis of coal tar by John
Kidd (1775–1851), a British physician and chemist, and Alexander Garden (1757–1829), an
American living in Britain. Kidd described the properties of the white crystals he obtained
from coal tar and proposed the named naphthaline for the substance; naphthaline was
derived from naphtha, a general term for a volatile, fl ammable, hydrocarbon liquid. Michael
Faraday (1791–1867) determined the correct empirical formula for naphthalene in 1825,
and Richard August Carl Emil Erlenmeyer (1825–1909) proposed the fused benzene ring
structure in 1866.
調製方法
Naphthalene is produced from coal tar or petroleum. It is made from petroleum by dealkylationof methylnaphthalenes in the presence of hydrogen at high temperature and pressure.Petroleum was a major source of naphthalene until the 1980s, but now most naphthaleneis produced from coal tar. The pyrolysis of bituminous coal produces coke and coke ovengases. Naphthalene is condensed by cooling the coke gas and then separated from the gas.
定義
naphthalene: A white volatilesolid, C
10H
8; r.d. 1.025;m.p. 80.55°C; b.p. 218°C. Naphthaleneis an aromatic hydrocarbon withan odour of mothballs and is obtainedfrom crude oil. It is a raw materialfor making certain syntheticresins.
一般的な説明
Heterogeneous ozonolysis of naphthalene adsorbed on XAD-4 resin has been studied using annular denuder technique.
空気と水の反応
Highly flammable. Insoluble in water.
反応プロフィール
Vigorous reactions, sometimes amounting to explosions, can result from the contact between aromatic hydrocarbons, such as Naphthalene, and strong oxidizing agents. They can react exothermically with bases and with diazo compounds. Substitution at the benzene nucleus occurs by halogenation (acid catalyst), nitration, sulfonation, and the Friedel-Crafts reaction. Naphthalene, camphor, glycerol, or turpentine will react violently with chromic anhydride [Haz. Chem. Data 1967. p 68]. Friedel-Crafts acylation of Naphthalene using benzoyl chloride, catalyzed by AlCl3, must be conducted above the melting point of the mixture, or the reaction may be violent [Clar, E. et al., Tetrahedron, 1974, 30, 3296].
危険性
Toxic by inhalation. Upper respiratory tract
irritant, cataracts and hemolytic anemia. Possible
carcinogen.
火災危険
Flammable/combustible material. May be ignited by friction, heat, sparks or flames. Some may burn rapidly with flare burning effect. Powders, dusts, shavings, borings, turnings or cuttings may explode or burn with explosive violence. Substance may be transported in a molten form at a temperature that may be above its flash point. May re-ignite after fire is extinguished.
安全情報
労働安全衛生法で「特定化学物質第2類物質、特定第2類物質」に該当します。船舶安全法、航空法においては可燃性物質類・可燃性物質となっています。皮膚や目に対して刺激性があるため、取り扱いには手袋や保護メガネなどの適切な保護具を着用する必要があります。油性溶剤や有機溶剤と混合すると引火性の高い混合物となるため、火気を避ける必要があります。
使用用途
ナフタリンは主に防虫剤、染料、医薬品、農薬、潤滑剤などの原料として使用されます。石油や石炭の乾留から得られる副産物であり、これらの産業の発展に貢献してきました。ナフタリンを原料とする染料は、耐久性が高く鮮やかな色合いが得られるため、綿織物や毛織物の染色に広く使用されています。医薬品の原料としても使用され、特に患者の血液中にあるフェノール類の検査に使用される試薬の1つとしても知られています。
ナフタリンを原料とする防虫剤は、農業用途や木材の防腐剤、衣料品の虫よけ剤などに幅広く利用されています。農薬としては、稲のカメムシ、施設野菜のウリハムシ、アザミウマ等の駆除に使用されますが、 登録失効農薬成分です。繊維の防虫剤としては、家庭での使用が一般的ですが、クリーニング業者などでも使用される場合があります。
置換体
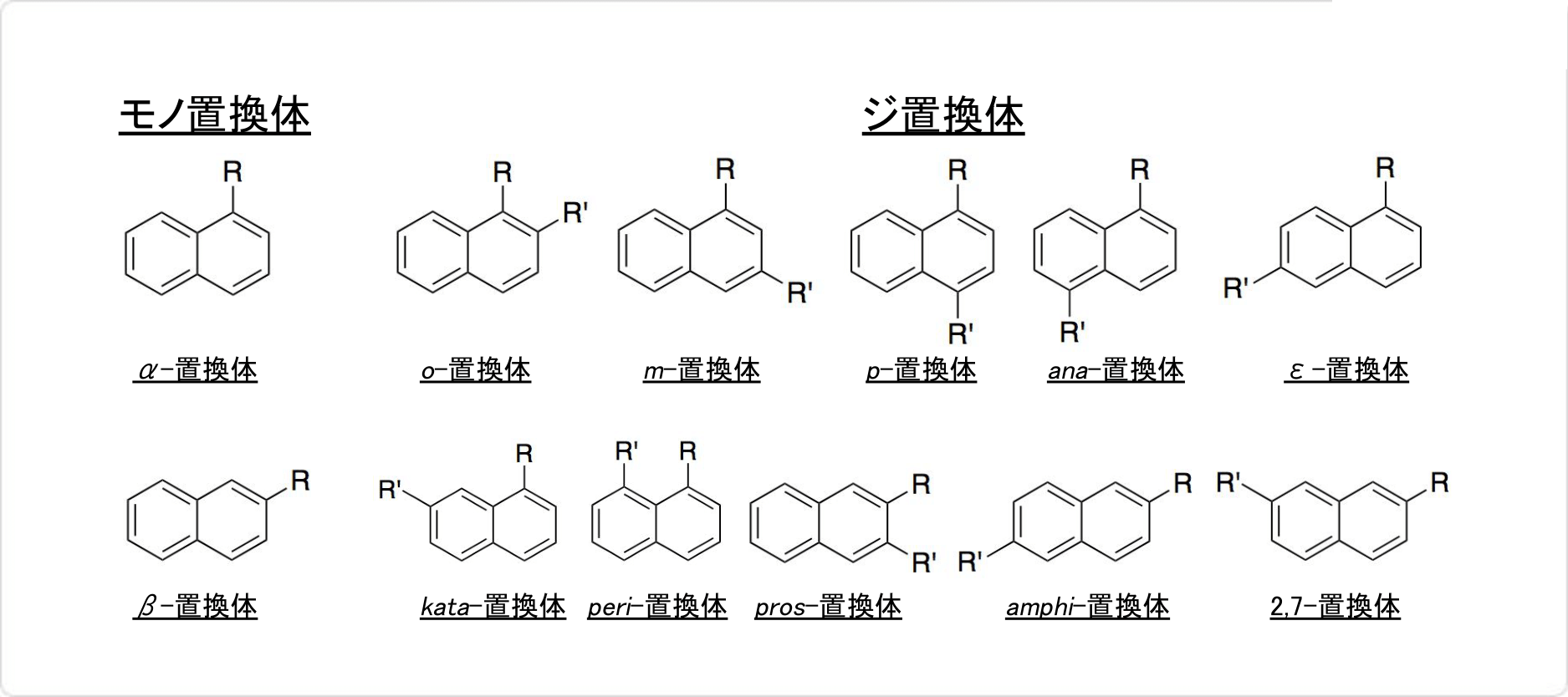
図3. ナフタレンの置換体
ナフタレンにはモノ置換体が2種類、ジ置換体が10種類存在します。2,7位ジ置換体以外には、すべて置換位置を示す接頭辞が付けられています。
1、4、5、8位のモノ置換体は、アルファ (α-) 、2、3、6、7位のモノ置換体は、ベータ (β-) です。1,2位のジ置換体はオルト (o-) 、1,3位のジ置換体はメタ (m-) 、1,4位のジ置換体はパラ (p-) 、1,5位のジ置換体はアナ (ana-) 、1,6位のジ置換体はエピ (ε-) 、1,7位のジ置換体はカタ (kata-) 、1,8位のジ置換体はペリ (peri-) 、2,3位のジ置換体はプロス (pros-) 、2,6位のジ置換体はアンフィ (amphi-) と付けられています。
使用用途
ナフタレンは室温でも揮発性に優れ、防虫剤や防臭剤として生活に広く使用されています。また、ナフタレンから生成されるは、ポリエステル繊維の原料としても使用可能です。
ナフタレンは、他にも染料中間体や合成樹脂、殺虫剤、滅菌剤、燃料、界面活性剤、ゴムに柔軟性を与える乳化重合用、有機顔料等の有機化学合成の原料としても幅広く用いられています。
ナフタレンを水素化すると、化学合成やインク塗料等の製造に有用な溶剤であるやデカリンを生成可能です。
置換基
ナフタレンから水素を1つ取り去った置換基は、ナフチル基 (英: naphthyl group) と呼ばれています。水素の位置によって、1-ナフチル基と2-ナフチル基が存在します。
安全性プロファイル
Human poison by
ingestion. Experimental poison by ingestion, intravenous, and intraperitoneal routes.
Moderately toxic by subcutaneous route. An
experimental teratogen. Experimental
reproductive effects. An eye and skin
irritant. Can cause nausea, headache,
daphoresis, hematuria, fever, anemia, liver
damage, vomiting, convulsions, and coma.
Poisoning may occur by ingestion of large
doses, inhalation, or skin absorption.
Questionable carcinogen with experimental
tumorigenic data. Flammable when exposed
to heat or flame; reacts with oxidizing
materials. Explosive reaction with dinitrogen
pentaoxide. Reacts violently with CrOs,
aluminum chloride + benzoyl chloride. Fires
in the benzene scrubbers of coke oven gas
plants have been attributed to oxidation of
naphthalene. Explosive in the form of vapor
or dust when exposed to heat or flame. To
fight fire, use water, CO2, dry chemical.
When heated to decomposition it emits
acrid smoke and irritating fumes.
職業ばく露
Naphthalene is used as a chemical
intermediate or feedstock for synthesis of phthalic, anthranilic,
hydroxyl (naphthols), amino (naphthylamines), and sulfonic
compounds; which are used in the manufacture of
various dyes and in the preparation of phthalic anhydride, 1-naphthyl-N-methyl carbonate; and β-naphthol. Naphthalene
is also used in the manufacture of hydronaphthalenes, synthetic
resins; lampblack, smokeless powder; and celluloid.
Naphthalene has been used as a moth repellent.
Approximately 100 million people worldwide have G6PD
deficiency which would make them more susceptible to
hemolytic anemia on exposure to naphthalene. At present,
more than 80 variants of this enzyme deficiency have been
identified. The incidence of this deficiency is 0.1% in
American and European Caucasians, but can range as high
as 20% in American blacks and greater than 50% in certain
Jewish groups. Newborn infants have a similar sensitivity
to the hemolytic effects of naphthalene, even without
G6PD deficiency.
発がん性
Naphthalene is reasonably anticipated to be a human carcinogenbased on sufficient evidence from studies in experimental animals.
輸送方法
UN1334 Naphthalene, crude or Naphthalene,
refined, Hazard Class: 4.1; Labels: 4.1-Flammable solid.
UN2304 (molten) Hazard Class: 4.1; Labels: 4.1-Flammable
solid.
純化方法
Crystallise naphthalene once or more times from the following solvents: EtOH, MeOH, CCl4, *C6H6, glacial acetic acid, acetone or diethyl ether, followed by drying at 60o in an Abderhalden drying apparatus. It has also been purified by vacuum sublimation and by fractional crystallisation from its melt. Other purification procedures include refluxing in EtOH over Raney Ni and chromatography of a CCl4 solution on alumina with *benzene as eluting solvent. Baly and Tuck [J Chem Soc 1902 1908] purified naphthalene for spectroscopy by heating with conc H2SO4 and MnO2, followed by steam distillation (repeating the process), and formation of the picrate which, after recrystallisation (m 150o) is decomposed with base and the naphthalene is steam distilled. It is then crystallised from dilute EtOH. It can be dried over P2O5 under vacuum (take care not to make it sublime). Also purify it by sublimation and subsequent crystallisation from cyclohexane. Alternatively, it has been washed at 85o with 10% NaOH to remove phenols, with 50% NaOH to remove nitriles, with 10% H2SO4 to remove organic bases, and with 0.8g AlCl3 to remove thianaphthalenes and various alkyl derivatives. Then it is treated with 20% H2SO4, 15% Na2CO3 and finally distilled. [Gorman et al. J Am Chem Soc 107 4404 1985.] Zone refining purified naphthalene from anthracene, 2,4-dinitrophenylhydrazine, methyl violet, benzoic acid, methyl red, chrysene, pentacene and indoline. [Beilstein 5 IV 1640.]
不和合性
Dust may form explosive mixture with
air. Incompatible with oxidizers (chlorates, nitrates, peroxides,
permanganates, perchlorates, chlorine, bromine, fluorine,
etc.); contact may cause fires or explosions. Keep
away from alkaline materials, strong bases, strong acids,
oxoacids, epoxides. Violent reactions with chromium(III)
oxide, dinitrogen pentoxide; chromic anhydride.
廃棄物の処理
Dissolve or mix the material
with a combustible solvent and burn in a chemical incinerator
equipped with an afterburner and scrubber. All federal,
state, and local environmental regulations must be
observed. Consult with environmental regulatory agencies
for guidance on acceptable disposal practices. Generators
of waste containing this contaminant (≥100 kg/mo) must
conform with EPA regulations governing storage, transportation,
treatment, and waste disposal.
参考文献
Formation Mechanisms of Naphthalene and Indene: From the Interstellar Medium to Combustion Flames. DOI:
10.1021/acs.jpca.6b09735Functional Naphthalene Diimides: Synthesis, Properties, and Applications. DOI:
10.1021/ACS.CHEMREV.6B00160The 100 Most Important Chemical Compounds: A Reference Guide DOI:
10.5860/choice.45-3798A comprehensive guide to the hazardous properties of chemical substances DOI:
10.1002/9780470134955A Dictionary of Chemistry (6th edition) DOI:
10.1108/09504120910935291TOXICOLOGICAL REVIEW of NAPHTHALENE Naphthalene - Environmental Protection Agency
ナフタレン 上流と下流の製品情報
原材料
準備製品
1,4,5,8-ナフタレンテトラカルボン酸
1-ナフトール
8-アミノ-1-ナフタレンスルホン酸
ジベンゾ[b,def]クリセン-7,14-ジオン
ナフタレンスルホン酸, メチレンビス, ジナトリウム
ビオラントレン-5,10-ジオン
α-リノレン酸
ブチルナフタレンスルホン酸ナトリウム
1-ニトロナフタレン
1,5-ジヒドロキシナフタレン
Δ2,2'(1H,1'H)-ビナフト[2,1-b]チオフェン-1,1'-ジオン
タンニン酸
屈班児歔伺磨欝
2,8-ジフェニルアントラ[2,1-d:6,5-d']ビスチアゾール-6,12-ジオン
SEC-BUTYLAMINE
1-ナフタレン酢酸
2-アミノナフタレン-4,8-ジスルホン酸
ジメチルナフタレン
イソペンタン
dispersing agent CNF
5-アミノ-2-ナフタレンスルホン酸
ナフテン酸
Dispersing agent DN
フタル酸無水物
5-アミノ-1-ナフタレンスルホン酸
Slushing agent,high efficiency
2-ナフタレンスルホン酸 ホルマリン
1-ヨードナフタレン
ナフタレン-1,5-ジスルホン酸
安息香酸
1-ナフチルアミン
Slushing agent
synthetic fiber oil QDC-201
2-ナフトール
5-ヒドロキシ-1-ナフタレンスルホン酸
1'-アセトナフトン