塩化バリウム 化学特性,用途語,生産方法
外観
白色, 顆粒~粉末
種類
市販されている塩化バリウムの製品には、研究開発用試薬製品や工業用薬品があります。どちらの場合も、塩化バリウム二水和物として販売されている場合が最も多く、メーカーによっては少数ながら無水物や一水和物も販売されています。
研究開発用試薬では、塩化バリウム二水和物として販売されている場合が多いです。少数ながら無水物製品も販売されている他、10w/v%の溶液として販売されている場合もあります。
純粋な物質では、容量の種類は25g、100g、500gなどで、主に実験室で取り扱いやすい容量での提供が一般的です。室温で保管可能な試薬製品とされることが多いです。
定義
本品は、バリウムの塩化物であり、次の化学式で表される。
性質
化学式 BaCl2 。塩化バリウムは,苦みがかった塩味のある無色の物質。飽和溶液から常温で無色の扁平(へんぺい)な結晶、または粉末状の二水和物が析出する。二水和物はわずかに吸湿性をもつ。これは121℃で無水和物に変わる。無水和物は常温では単斜晶系の結晶であるが、転移点925℃で等軸晶系に変わる。水に溶けやすいが、エタノール(エチルアルコール)やアセトンには溶けない。レーキ顔料、バリウム塩の原料、ボイラー用水の軟化剤などのほか、分析試薬としても用いられる。
溶解性
水に易溶, メタノールに可溶。
解説
BaCl2(208.24).炭酸バリウムを塩酸に溶かして濃縮すると二水和物が得られる.二水和物を加熱すると一水和物を経て113 ℃ で無水物となる.無水物は低温で単斜晶系.密度3.89 g cm-3.925 ℃ で等軸晶系に転移する.融点963 ℃,沸点1560 ℃.水によく溶けるが潮解性はない.二水和物は無色の偏平な四角形の単斜晶系結晶.密度3.097 g cm-3.水に易溶,エタノールに難溶,アセトンに不溶.レーキ顔料,金属の熱処理剤,バリウム塩の製造,殺そ剤,殺虫剤,皮なめしなどに用いられる.分析試薬として硫酸イオンの定性および定量に重要である.有毒.[CAS 10361-37-2:BaCl2][CAS 10326-27-9:BaCl2・2H2O]
森北出版「化学辞典(第2版)
用途
レーキ顔料,媒染剤,殺虫剤など
生産
化学式BaCl2。重晶石BaSO4の粉末,木炭,塩化カルシウムの混合物を赤熱してから熱湯で抽出し,不純物を除いたのち結晶を析出させると得られる。 BaSO4+4C+CaCl2 ―→BaCl2+CaS+4CO炭酸バリウムと塩酸の反応でも得られる。飽和水溶液から常温で析出するのは2水和物BaCl2・2H2Oで,これは平たい四角形の無色結晶である。125℃で無水和物BaCl2となり,中間の温度(60℃)では1水和物BaCl2・H2Oが生ずる。以前は重晶石(硫酸バリウム)を塩化カルシウムと溶融して製造していたが、現在では硫化バリウム溶液と塩酸との反応によっている。
合成
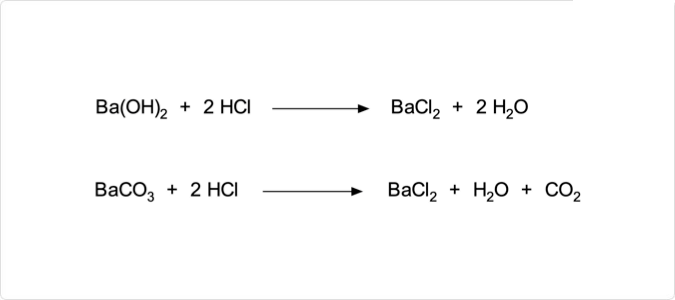
図1. 塩化バリウムの合成方法
塩化バリウムは、水酸化バリウムや炭酸バリウムと塩酸を反応させることによって合成が可能です。工業的な製造方法としては、重晶石 () をと溶融する方法や、とを反応させる方法などが用いられています。
説明
Barium dichloride is a white solid, odorless, hygroscopic chemical substance. Barium
dichloride is used in the manufacture of pigments, in the manufacture of other barium
salts and in fireworks to give a bright green color. It is one of the most common watersoluble
salts of barium. Like other barium salts, it is toxic and imparts a yellow-green coloration
to a flame. Barium chloride has wide application in the laboratory.
化学的特性
Barium chloride,BaCI2, is a colorless toxic salt with a melting point of 963°C. It is soluble in water. Barium chloride is used in metal surface treatment and as a rat poison.
物理的性質
Barium chloride has the formula, BaCl2 and is an
ionic chemical compound. It is one of the most important
water-soluble salts of barium-containing
compounds. Like other barium salts, it is toxic and
imparts a yellow-green coloration to a flame. It is
also hygroscopic. Barium chloride was the by-product
of the discovery of radium by Madame Curie (1898).When refining radium, the final separation resulted in
barium chloride and radium chloride.
BaCl2 crystallizes in both the cubic “fluorite” and
“lead chloride” crystal structures, both of which accommodate
the preference of the large Ba
2+ ion for coordination
numbers greater than six.
製造方法
Barium chloride can be prepared from barium
hydroxide or barium carbonate, the latter being found
naturally as the mineral “Witherite”. These basic salts
react to give hydrated barium chloride. On an industrial
scale, it is prepared via a two-step process from the
mineral “Baryte”:
BaSO4+4C→BaS+4CO (gas)
This first step requires high temperatures. The second
step requires fusion of the reactants:
BaS+ CaCl2→BaCl2+CaS
The BaCl2 is then be leached out from the mixture
with water. From water solutions of barium chloride,
the dihydrate can then be crystallized as white crystals,
BaCl2·2H2O, which are colorless, translucent rhomboidal
tablets or lamellae. The dihydrate is stable in
the air at room temperature, but loses one-half of its
water above 55°C(131F), and becomes anhydrous at
121°C (250 F).
一般的な説明
Any of a variety of substances that contain barium. Most are whitish colored crystalline solids. They tend to be soluble in water and denser than water. They may be toxic by inhalation or possibly skin absorption. They are often used to make other chemicals.
空気と水の反応
Water soluble.
反応プロフィール
BARIUM CHLORIDE may react violently with BrF3 and 2-furan percarboxylic acid in its anhydrous form.
危険性
Ingestion of 0.8 g may be fatal.
火災危険
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
化学性质
融点962℃,沸点1560℃
法規制
塩化バリウムは有害な物質であるため、各種法令による規制の対象となっています。例えば、労働安全衛生法においては、名称を表示すべき危険有害物、、名称を通知すべき危険有害物、及びリスクアセスメントを実施すべき危険有害物に指定されています。
また、毒物・劇物取締法における劇物です。法令を遵守して正しく取り扱うことが重要です。
使用用途
塩化バリウムの主な使用用途は、有機顔料、製紙充填剤、金属熱処理剤、レントゲン造影剤原料、蛍光体原料などです。
1. バリウム塩原料
最も広く用いられている用途は、他のバリウム塩の原料としての使用です。例えば、塗料、ゴムなどの増量剤やX線造影剤として知られている硫酸バリウムは、工業的には重晶石から得られた硫化バリウムをと反応させて得られます。塩化バリウムの水溶液と硫酸ナトリウムを用いて共沈反応によって製造する手法も有効です。
2. 硫酸塩の比濁分析
硫酸塩の比濁分析も塩化バリウムの重要な用途の1つです。比濁分析とは、塩化バリウムが硫酸イオンと反応して不溶性の硫酸バリウムを生成する反応を利用して、硫酸イオンの定性および定量分析を行う手法です。
工業用途
Barium chloride (BaCl
2·2H
2O) is a colorless, white powder highly soluble in water (25%
at 10 °C). It is quite a toxic reagent. Barium chloride is used during borite flotation as an
activator. Barium chloride also has a depressing effect on fluorite and cassiterite.
安全性プロファイル
A poison by ingestion,
subcutaneous, intravenous, and
intraperitoneal routes. Inhalation absorption
of barium chloride equals 60-80%; oral
absorption equals 10-30%. Experimental
reproductive effects. Mutation data
reported. See also BARIUM
COMPOUNDS (soluble). When heated to
decomposition it emits toxic fumes of Cl-.
合成方法
炭酸バリウムを塩酸に溶かし,濃縮して得られる二水和物を125℃以上で加熱する
参考文献
E.B. Brackett, T.E. Brackett, R.L. Sass, J. Phys. Chem., 67, 2132 (1963), DOI: 10.1021/j100804a038.
塩化バリウム 上流と下流の製品情報
原材料
準備製品