ジメチル硫酸 化学特性,用途語,生産方法
外観
無色〜うすい黄色, 澄明の液体
性質
硫酸ジメチルの融点は−32°Cで、188°Cで分解します。タマネギのような弱い悪臭を有し、無色の油状液体です。、アセトン、ジクロロメタン、各種エーテル類に溶けます。水には溶けにくく、徐々に加水分解されます。
硫酸ジメチルには刺激性がないですが、人体にきわめて有毒です。蒸気を吸入したり、皮膚に付着したまま放置すると、体内に吸収されて中毒を起こします。腎臓、肝臓、心臓が侵され、皮膚の壊死や呼吸器粘膜の炎症から死に至る可能性もあるため、取り扱いには十分な注意が必要です。
溶解性
水と反応。エタノール, エーテル, アセトンと混和。エタノール及びアセトンに極めて溶けやすく、水にほとんど溶けない。
解説
ジメチル硫酸,メチルアルコールあるいはジメチルエーテルと発煙硫酸から得られる.ジメチル硫酸はい無色の油状の液体.融点-27 ℃,沸点188 ℃,76 ℃(2 kPa).d204 1.3322.n204 1.3874.もっとも効果的なメチル化剤の一種である.[CAS 77-78-1]
森北出版「化学辞典(第2版)
用途
合成剤、溶剤、安定剤
用途
ジメチル硫酸使用されます有機合成のメチル化剤、中間物アニソール、香料ネロリンの合成、医薬品(ピリン剤、カフェイン、ビタミン等)の合成、メチルハイドロキノンやポリメチシアニン染料、メチルセルロースの製造、芳香族炭化水素の抽出用溶剤、安定剤(無水硫酸、ジシアノエチレンモノマー)
構造
硫酸ジメチルは中性エステルであり、化学式は(CH3)2SO4または(CH3O)2SO2で表されます。Me2SO4とも表記されます。モル質量は126.13g/molで、密度は1.33g/mlです。
毒性
ジメチル硫酸毒性が強,皮膚から吸収され,壊死を起こし,粘膜,とくに呼吸器粘膜の炎症を起こす.腎臓,肝臓,心臓もおかされる.LD50 205 mg/kg(ラット,経口).
森北出版「化学辞典(第2版)
合成法
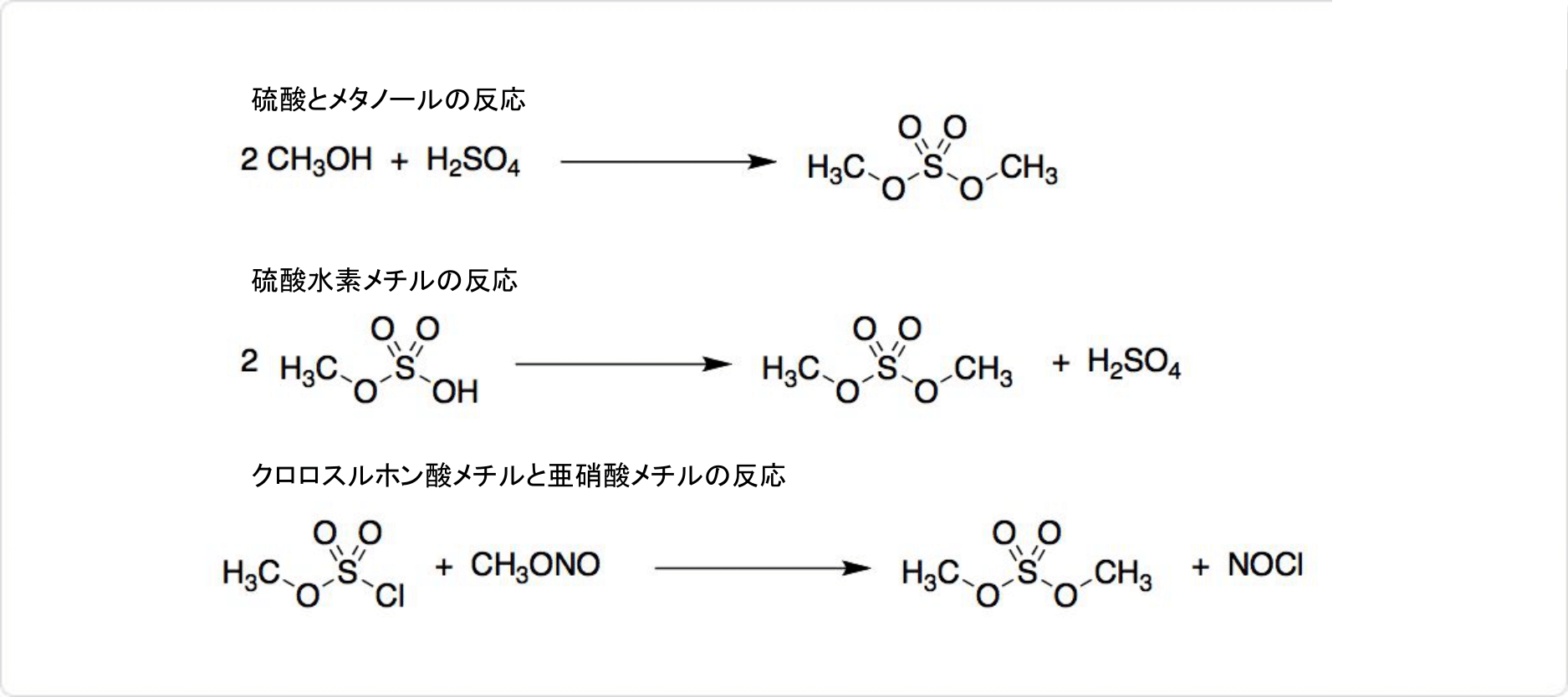
図2. 硫酸ジメチルの合成
発煙硫酸と乾いたやと反応させると、硫酸水素メチルが生成します。硫酸水素メチルを蒸留し、硫酸ジメチルに変換可能です。
クロロスルホン酸メチルと亜硝酸メチルを反応させても、硫酸水素メチルを合成可能です。
工業的にはジメチルエーテルと (SO3) との連続反応によって合成されます。
危険性
ラットに対して吸入や静脈内投与によって、がんの発生が確認されています。有毒で取り扱いにくいため、実験室では他のメチル化剤に代替される傾向があります。
ヨウ化メチルはO-メチル化に利用され、高価ですが、危険性が低いです。炭酸ジメチルはヨウ化メチルや硫酸ジメチルよりも毒性が低いため、N-メチル化に硫酸ジメチルの代わりとして使用可能です。ただし硫酸ジメチルは反応効率が高く、入手が容易なため、適切な試薬に判断される場合もあります。
説明
Dimethyl sulfate is a colorless, oily liquid with a faint, onionlike
odor. It is soluble in water, ether, dioxane, acetone,
benzene, and other aromatic hydrocarbons, miscible with
ethanol, and sparingly soluble in carbon disulfide. It is stable
under normal temperatures and pressures, but hydrolyzes
rapidly in water at or above 18 ℃.
Dimethyl sulfate has been produced commercially since at
least the 1920s. One production method is continuous reaction
of dimethyl ether with sulfur trioxide. In 2009, dimethyl sulfate
was produced by 33 manufacturers worldwide, including 1 in
the United States, 14 in China, 5 in India, 5 in Europe, 6 in East
Asia, and 2 in Mexico, and was available from 44 suppliers,
including 16 US suppliers. There are no data on US imports or
exports of dimethyl sulfate. Reports filed from 1986 through
2002 under the US Environmental Protection Agency’s Toxic
Substances Control Act Inventory Update Rule indicate that US
production plus imports of dimethyl sulfate totaled 10–50
million pounds. The simplest way of synthesizing dimethyl
sulfate is by esterification of sulfuric acid with methanol as
follows:2CH3OH+ H2SO4→(CH3)2SO4 + 2H2O
化学的特性
Dimethyl sulfate is essentially odorless. The specific gravity of this colorless, corrosive, oily liquid is 1.3322 g/cm3. Dimethyl sulfate is soluble in ether, dioxane, acetone, benzene, and other aromatic hydrocarbons. It is sparingly soluble in carbon disulfide and aliphatic hydrocarbons, and only slightly soluble in water (28 g/l at 18 °C) (O'Neil, 2006).
使用
Dimethyl sulfate is a strong alkylating agent and might also react with the carboxylic acid substrate, further reducing the DMS concentration in the mixture. It is used as a methylating agent in themanufacture of many organic compounds,such as, phenols and thiols. Also, it is used inthe manufacture of dyes and perfumes, andas an intermediate for quaternary ammoniumsalts. It was used in the past as a militarypoison.
主な応用
Dimethyl Sulfate is a diester of methanol and sulfuric acid. Dimethyl Sulfate is commonly used as a reagent for the methylation of phenols, amines, and thiols. Dimethyl Sulfate is an effective and widely used probe for sequence-specific protein-DNA interactions.
定義
ChEBI: Dimethyl sulfate is the dimethyl ester of sulfuric acid. It has a role as an alkylating agent and an immunosuppressive agent.
製造方法
Dimethyl sulfate is prepared by distillation of an oleum/methanol mixture; technical production using dimethyl ether and SO3 has also been reported (NLM, 2013).
一般的な説明
Dimethyl sulfate is a colorless oily liquid, odorless to a faint onion-like odor. Dimethyl sulfate is very toxic by inhalation. Dimethyl sulfate is a combustible liquid and has a flash point of 182°F. Dimethyl sulfate is slightly soluble in water and decomposed by water to give sulfuric acid with evolution of heat. Dimethyl sulfate is corrosive to metals and tissue.
空気と水の反応
Water soluble.
反応プロフィール
Pure Dimethyl sulfate and concentrated aqueous ammonia react extremely violently with one another, as is the case for tertiary organic bases, [NFPA 491M, 1991]. Dimethyl sulfate ignites in contact with unheated barium chlorite, due to the rapid formation of unstable methyl chlorite. The product of methylating an unnamed material at 110°C was alloyed to remain in a reactor for 80 min. before the reactor exploded. This involved a sulfur ester such as Dimethyl sulfate, [MCA Case History No. 1786].
健康ハザード
Dimethyl sulfate is extremely hazardous because of its lack of warning properties and
delayed toxic effects. The vapor of this compound is extremely irritating to the skin, eyes,
and respiratory tract, and contact with the liquid can cause very severe burns to the eyes
and skin. Ingestion of dimethyl sulfate causes burns to the mouth, throat, and
gastrointestinal tract. The effects of overexposure to dimethyl sulfate vapor may be
delayed. After a latent period of 10 hours or more, headache and severe pain to the eyes
upon exposure to light may occur, followed by cough, tightness of the chest, shortness of
breath, difficulty in swallowing and speaking, vomiting, diarrhea, and painful urination.
Fatal pulmonary edema may develop. Systemic effects of dimethyl sulfate include
damage to the liver and kidneys.
Dimethyl sulfate is listed by IARC in Group 2A ("probable human carcinogen") and is
classified as a "select carcinogen" under the criteria of the OSHA Laboratory Standard.
Data indicate that dimethyl sulfate does not specifically harm unborn animals; dimethyl
sulfate is not a developmental toxin. It is a strong alkylating agent and does produce
genetic damage in animals and in bacterial and mammalian cell cultures.
燃焼性と爆発性
Dimethyl sulfate is a combustible liquid (NFPA rating = 2). Toxic dimethyl sulfate vapors are produced in a fire. Carbon dioxide or dry chemical extinguishers should be used to fight dimethyl sulfate fires.
使用用途
硫酸ジメチルは強力なアルキル化剤として広く用いられています。そのため、医薬品原料や合成原料としての用途が多いです。具体的には、やアルコールのヒドロキシ基 (-OH) 、アミンのアミノ基 (-NH2) 、チオールのチオール基 (-SH) のメチル化によく用いられます。
核酸の化学的メチル化に用いられるアルキル化剤としても有名です。歴史的に、マクサム・ギルバート法 (英: Maxam–Gilbert sequencing) によるDNA塩基配列の決定に使用されました。
化学性质
沸点72.5~72.7℃(13mmHg)
化学反応
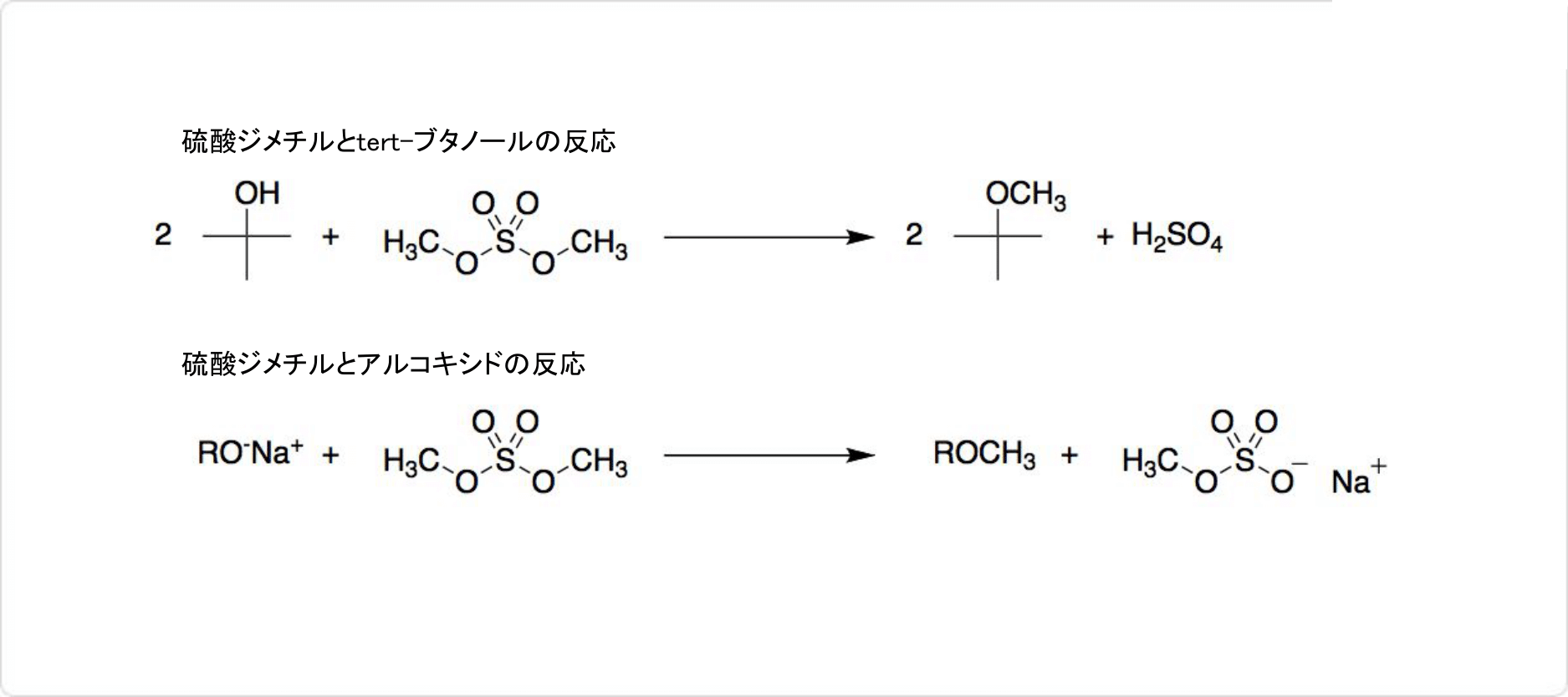
図3. 硫酸ジメチルの反応
一般的に硫酸ジメチルは、フェノール類をメチル化する際に使用され、アルコールのメチル化にも適しています。例えば、tert-ブタノールからtert-ブチルメチルエーテルを合成可能です。アルコキシドの場合には、すぐにメチル化します。アルコールと同様に、チオールのメチル化も容易に起こります。チオカルボン酸からチオエステルを生成可能です。
アミンのメチル化によって、3級アミンや4級アンモニウム塩を合成できます。長鎖アルキル基を有する4級アンモニウム化合物は、衣類の柔軟剤や界面活性剤に使用可能です。
さらに硫酸ジメチルは、グアニンのイミダゾール環の開環反応によって、DNAを塩基特異的に開裂でき、DNA鎖の切断や塩基配列の決定で役立ちます。また酸化銅(I)と反応すると、硫酸銅(I)が得られます。
発がん性
Dimethyl sulfate is reasonably anticipated to be a human carcinogenbased on sufficient evidence of carcinogenicity from studies in experimental animals.
環境運命予測
Chemical/Physical. Hydrolyzes in water (half-life = 1.2 h) to methanol and sulfuric acid
(Robertson and Sugamori, 1966) via the intermediate methyl sulfuric acid (Du Pont, 1999a)
貯蔵
work with dimethyl sulfate should be conducted in a fume hood to prevent exposure by inhalation, and appropriate impermeable gloves and safety goggles should be worn at all times to prevent skin and eye contact.
不和合性
Dimethyl sulfate can react violently with ammonium hydroxide, sodium azide, and strong oxidizers.
廃棄物の処理
Excess dimethyl sulfate and waste material containing this substance should be placed in a covered metal container, clearly labeled, and handled according to your institution's waste disposal guidelines.
ジメチル硫酸 上流と下流の製品情報
原材料
準備製品
Cationic Brilliant Blue RL
2,4,5-Trimethoxynitrobenzene
3'-ジメチルアミノアセトフェノン
3,5-ジブロモ-2-メトキシベンズアルデヒド
4-クロロ-2,5-ジメトキシアニリン
3,4,5-トリメトキシベンズヒドラジド
3-ジメチルアミノ安息香酸
フルスルチアミン
アミノピリン
1,2-ジベンゾイル-1,2-ジメチルヒドラジン
2-(4-(ACETYLAMINO)PHENYL)PROPIONITRILE
6-AMINO-2-METHYLTHIO-3-METHYLURACIL
o-アニス酸
3,5-ジメトキシ安息香酸
2-(4-ニトロフェニル)プロピオノニトリル
ダンシル酸
6,7-ジメトキシクマリン
4-アミノ-α-メチルベンゼンアセトニトリル
antistatic Agent TM
Cationic surface active agent
trimethyl lauroylaminopropyl ammonium methylsulfate
2,4-ジメトキシ安息香酸
ビフェナゼト
4-メチルジベンゾチオフェン
α-Dimethoxymethyl-methoxypropionitrile
4-(アセチルアミノ)-5-クロロ-2-メトキシ安息香酸メチル
2-メトキシ-4-(アセチルアミノ)安息香酸メチル
1-CHLOROMETHYL-2,3,4-TRIMETHOXYBENZENE
3,4-ジヒドロキシ-5-メトキシ安息香酸メチルエステル
2'-(メチルアミノ)-5'-クロロベンゾフェノン
2-アミノ-7-メチルヒポキサンチン
2-(メチルチオ)安息香酸
softening CS
(3R,8aβ)-2,3,4,5,6,7,8,8a-オクタヒドロ-6-メトキシ-3β,6β,8,8-テトラメチル-1H-3aα,7α-メタノアズレン
1-メチル-5-ニトロ-1H-イミダゾール
2-(4-ACETAMINO-3-CHLOROPHENYL)PROPIONITRILE
トリフルオロメタンスルホン酸メチル
7-メトキシ-3,4,5,6-テトラヒドロ-2H-アゼピン
オキシベンゾン
5-クロロ-2-メトキシ安息香酸