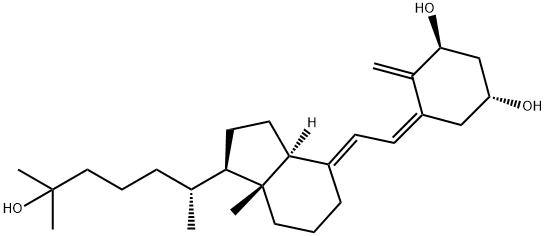
Calcitriol
|
|
Calcitriol 속성
- 녹는점
- 119-1210C
- 알파
- D25 +48° (methanol)
- 끓는 점
- 474.91°C (rough estimate)
- 밀도
- 1.0362 (rough estimate)
- 굴절률
- 1.4700 (estimate)
- 인화점
- 14 °C
- 저장 조건
- 2-8°C
- 산도 계수 (pKa)
- 14.43±0.40(Predicted)
- 물리적 상태
- 고체
- 물리적 상태
- 단단한 모양
- 색상
- 흰색에서 거의 흰색
- 최대 파장(λmax)
- 265nm(lit.)
- Merck
- 14,1644
- BRN
- 7559394
- BCS Class
- 2,4
- 안정성
- 공기와 빛에 민감함
- InChIKey
- GMRQFYUYWCNGIN-UNVJKIGWSA-N
- CAS 데이터베이스
- 32222-06-3(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | T+,Xn,F | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 26/27/28-63-36/37/38-20/21/22-11 | ||
| 안전지침서 | 36/37/39-45-36-26-16-7 | ||
| 유엔번호(UN No.) | UN 2811 6.1/PG 1 | ||
| WGK 독일 | 3 | ||
| RTECS 번호 | FZ4645000 | ||
| F 고인화성물질 | 8-10-19 | ||
| 위험 등급 | 6.1(a) | ||
| 포장분류 | I | ||
| HS 번호 | 29061900 | ||
| 유해 물질 데이터 | 32222-06-3(Hazardous Substances Data) | ||
| 독성 | mouse,LD50,intraperitoneal,1900ug/kg (1.9mg/kg),SENSE ORGANS AND SPECIAL SENSES: LACRIMATION: EYEBEHAVIORAL: ATAXIA,Yakuri to Chiryo. Pharmacology and Therapeutics. Vol. 11, Pg. 4175, 1983. |
Calcitriol C화학적 특성, 용도, 생산
개요
Calcitriol is the active form of vitamin D and is also a kind of hormones in the body. It plays an important role in the regulation of calcium and phosphorus concentration. It can increase the blood calcium level by increasing the intestinal absorption of calcium and can also increase the release of the bone calcium release for increasing the blood calcium levels. This study was first conducted and reported by Michael F. Holick in 1971. The basic principle of action model of calcitriol in many cases is through binding with the vitamin D receptor (the VDR), e.g., the ligand-receptor complex forming between calcitriol and its receptor in the intestinal epithelial cells cytoplasm can be transferred to the nucleus for being as the transcriptional factor in promoting the expression of calcium-binding protein. The increased level of calcium-binding proteins can help the cell to actively transport of more calcium ions, thereby increasing the calcium absorption level. Calcium absorption while maintaining electrical neutrality also requires transporting anions, mainly the absorption of inorganic phosphate ions, so calcitriol also promote the absorption of phosphorus.Clinically, this drug can be used in the treatment of hypocalcemia, hypoparathyroidism (adult), osteomalacia, rickets (infants), chronic kidney disease, renal osteodystrophy, osteoporosis, as well as the prevention of glucocorticoid induced osteoporosis.
Calcitriol-dimensional molecular structure
The above information is edited by the chemicalbook of Dai Xiongfeng.
화학적 성질
White Crystalline Powder용도
Calcitriol (1α,25-dihydroxy Vitamin D3) is synthesized from 7-dehydrocholesterol in humans via a non-enzymatic photochemical reaction with 290-310 nm UV light in the skin. Hydroxylation of the resulting cholecalciferol in the liver produces 25-hydroxy Vitamin D3, the principal circulating form of Vitamin D. A second, tightly regulated hydroxylation in the kidney produces calcitriol. Plasma calcitriol levels range from 10-70 pg/ml and are influenced by numerous dietary and hormonal factors. The main physiologic effects of calcitriol are to increase the absorption of calcium at the level of the intestinal epithelium, and to increase the mineralization of bone via the direct stimulation of osteoblasts.[Cayman Chemical]정의
ChEBI: A hydroxycalciol that is calcidiol in which the pro-S hydrogen of calcidiol is replaced by a hydroxy group. It is the active form of vitamin D3, produced fom calciol via hydoxylation in the li er to form calcidiol, which is subsequently oxidised in the kidney to give calcitriol.일반 설명
1α,25-Dihydroxyvitamin D3, also referred as calcitriol, is a calcitrophic hormone. It is the most biologically active metabolite of vitamin D produced in proximal tubular cells in the kidney.생물학적 활성
Active metabolite of vitamin D 3 that activates the vitamin D receptor (VDR). Displays calcemic actions; stimulates intestinal and renal Ca 2+ absorption and regulates bone Ca 2+ turnover. Exhibits antitumor activity; inhibits in vivo and in vitro cell proliferation in a wide range of cells including breast, prostate, colon, skin and brain carcinomas and myeloid leukemia cells.Calcitriol 준비 용품 및 원자재
원자재
준비 용품
Calcitriol 공급 업체
글로벌( 596)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Xi'an Yutbon Pharmaceutical Technology Co., Ltd | 029-81140587 +8618717328141 |
sales@yutbon.com | China | 437 | 58 |
| Finetech Industry Limited | +86-27-87465837 +8618971612321 |
info@finetechnology-ind.com | China | 9702 | 58 |
| HangZhou RunYan Pharma Technology Co.,LTD. | +86-88660901 +86-18112526015 |
sales@runyanpharma.com | China | 326 | 58 |
| ChemExpress | +86-021-58950125 |
info@chemexpress.com | China | 557 | 58 |
| Sigma Audley | +86-18336680971 +86-18126314766 |
nova@sh-teruiop.com | China | 525 | 58 |
| airuikechemical co., ltd. | +undefined86-15315557071 |
sales02@airuikechemical.com | China | 994 | 58 |
| Shanghai Aosiris new Material Technology Co., LTD | 86-15139564871 +8615139564871 |
wrjmoon2000@163.com | China | 352 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 |
sales@capotchem.com | China | 29797 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21691 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 |
fandachem@gmail.com | China | 9348 | 55 |
Calcitriol 관련 검색:
2-Keto-D-gulonic Acid Methyl Ester
6-(4-{2-[5-(tert-Butyl-dimethyl-silanyloxy)-2-methylene-cyclohexylidene]-ethylidene}-7a-methyl-octahydro-inden-1-yl)-2-methyl-heptan-2-ol
Calcium dobesilate
Tacrolimus
Calcitonin salmon
Ampicillin
SB216763
Bortezomib
Bosutinib
Alectinib
Dasatinib
Crizotinib
VitaMin D4
FALECALCITRIOL
Calcitriol
AURORA KA-6724
1ALPHA,25-DIHYDROXY[23,24(N)-3H]-CHOLECALCIFEROL
1ALPHA,25-DIHYDROXY[26,27-METHYL-3H]-CHOLECALCIFEROL