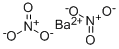질산 바륨
|
|
질산 바륨 속성
- 녹는점
- 592 °C (dec.)(lit.)
- 밀도
- 3.23
- 저장 조건
- Store at +5°C to +30°C.
- 용해도
- 94g/L
- 물리적 상태
- 고체
- 물리적 상태
- 단단한 모양
- Specific Gravity
- 3.24
- 색상
- 하얀색
- 수소이온지수(pH)
- 5.0-8.0 (50g/l, H2O, 25℃)
- 냄새
- 냄새 없는
- 수용성
- 9g/100mL(20℃)
- 감도
- Hygroscopic
- Merck
- 14,983
- Solubility Product Constant (Ksp)
- pKsp: 2.33
- 노출 한도
- ACGIH: TWA 0.5 mg/m3
NIOSH: IDLH 50 mg/m3; TWA 0.5 mg/m3
- Dielectric constant
- 5.8(0.0℃)
- 안정성
- 안정적인. 강산화제 - 가연성 물질과 접촉하면 화재가 발생할 수 있습니다. 가연성 물질, 환원제, 산, 산무수물과 혼합할 수 없습니다. 수분에 민감합니다.
- InChIKey
- IWOUKMZUPDVPGQ-UHFFFAOYSA-N
- CAS 데이터베이스
- 10022-31-8(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | O,Xn,Xi,C | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 8-20/22-36/38-34-26-25 | ||
| 안전지침서 | 28-28A-17-26-45-36/37/39-36-23 | ||
| 유엔번호(UN No.) | UN 1446 5.1/PG 2 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | CQ9625000 | ||
| TSCA | Yes | ||
| 위험 등급 | 5.1 | ||
| 포장분류 | II | ||
| HS 번호 | 28342990 | ||
| 유해 물질 데이터 | 10022-31-8(Hazardous Substances Data) | ||
| 독성 | LD50 i.v. in ICR mice: 20.10 mg Ba2+/kg (Syed, Hosain) | ||
| 기존화학 물질 | KE-02070 |
질산 바륨 C화학적 특성, 용도, 생산
개요
Barium nitrate is a stable, strong oxidiser. It is incompatible with combustible material, reducing agents, acids, acid anhydrides, and moisture-sensitive substance. Barium nitrate is poisonous, is a respiratory irritant, and is hazardous if mixed with flammable materials. Barium oxide plus zinc, aluminium and magnesium alloys are combustibles (paper, oil, wood), acids, and oxidisers and is hazardous. Mixtures with finely divided aluminium–magnesium alloys are easily ignitable and extremely sensitive to friction or impact. Barium nitrate mixed with aluminium powder, a formula for flash powder, is highly explosive. However, barium nitrate is noncorrosive in presence of glass. It is used in military thermite grenades, in the manufacturing process of barium oxide, in the vacuum tube industry, and in pyrotechnics for green flame.
화학적 성질
Barium nitrate is a shiny, white crystalline solid. It forms white crystals that are soluble in water at 20℃. It is formed by the reaction of barium carbonate or barium hydroxide with nitric acid. It is hazardous as magnesium plus barium oxide plus zinc, aluminum and magnesium alloys, combustibles (paper, oil, wood), acids, and oxidizers. Mixtures with nely divided aluminum-magnesium alloys are easily ignitable and extremely sensitive to friction or impact. Barium nitrate on contact with combustible materials will ignite. Barium nitrate mixed with aluminum powder, a formula for l ash powder is highly explosive.물리적 성질
Barium nitrate has the molecular formula of Ba(NO3)2 and the molecular weight of 261.3745 g/mol. It is also known as “nitrobarite”. Its CAS number is 10022-31-8. It is soluble in water.It can be prepared by a number of methods. The reaction between nitric acid and barium metal is one way and reaction with BaO or BaCO3 is another. Barium hydroxide and ammonium nitrate also form the product but ammonia is released as a by-product:
2HNO3 + Ba ---> Ba(NO3)2 +H2
2HNO3 + BaO ---> Ba(NO3)2 +H2O
Ba(OH)2 + 2NH4NO3 ---> Ba(NO3)2 + 2NH3 + 2H2O
Barium nitrate can also be prepared by the reaction of barium carbonate or barium carbonate with nitric acid:
BaCO3 + 2HNO3 ---> Ba(NO3)2 + CO2 +H2O
용도
Barium nitrate is used in industry in the production of green signal lights, to remove gases from vacuum tubes, and in the production of barium oxide.제조 방법
Barium Nitrate can be prepared by a number of methods. The reaction between nitric acid and barium metal is one way and reaction with BaO or BaCO3 is another. Barium hydroxide and ammonium nitrate also form the product but ammonia is released as a by-product:2HNO3+ Ba→Ba(NO3)2+H2
2HNO3+ BaO→Ba(NO3)2+H2O
Ba(OH)2+ 2NH4NO3→Ba(NO3)2+ 2NH3+ 2H2O
Barium nitrate can also be prepared by the reaction of barium carbonate or barium carbonate with nitric acid:
BaCO3+ 2HNO3→Ba(NO3)2+ CO2+H2O
In this method, barium carbonate is suspended in nitric acid. The solution is filtered and the product crystallizes out. Alternatively, barium carbonate and nitric acid are added to a saturated solution of barium nitrate. The product is then obtained by crystallization. Barium nitrate may also be prepared by adding sodium nitrate to a saturated solution of barium chloride. Barium nitrate precipitates out from the solution. The precipitate is filtered, washed with alcohol and dried.