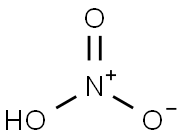
질산
|
|
질산 속성
- 녹는점
- -42 °C
- 끓는 점
- 120.5 °C(lit.)
- 밀도
- 1.41 g/mL at 20 °C
- 증기 밀도
- 1 (vs air)
- 증기압
- 8 mm Hg ( 20 °C)
- 인화점
- 120.5°C
- 저장 조건
- Store at +2°C to +25°C.
- 용해도
- 물과 섞일 수 있습니다.
- 산도 계수 (pKa)
- -1.3(at 25℃)
- 물리적 상태
- 액체, 이중 하위 끓는점 석영 증류
- 색상
- 무색~진한 노란색
- Specific Gravity
- d 20/4 1.4826
- 냄새
- <5.0ppm에서 감지 가능한 질식 연기
- pH 범위
- 1
- 수소이온지수(pH)
- 3.01(1 mM solution);2.04(10 mM solution);1.08(100 mM solution);
- 수용성
- >100g/100mL(20ºC)
- 감도
- Hygroscopic
- Merck
- 14,6577
- 노출 한도
- TLV-TWA 2 ppm (5 mg/m3) (ACGIH, MSHA, OSHA, and NIOSH); STEL 4 ppm (~10 mg/m3) (ACGIH).
- Dielectric constant
- 55.0(14℃)
- LogP
- -0.773 (est)
- CAS 데이터베이스
- 7697-37-2(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | C,O,Xi,T+ | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 8-35-34-20-41-37/38-36/38-26/27 | ||
| 안전지침서 | 23-26-36-45-36/37/39-39-60-28 | ||
| 유엔번호(UN No.) | UN 3264 8/PG 3 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | QU5900000 | ||
| F 고인화성물질 | 8 | ||
| TSCA | Yes | ||
| HS 번호 | 2808 00 00 | ||
| 위험 등급 | 8 | ||
| 포장분류 | II | ||
| 유해 물질 데이터 | 7697-37-2(Hazardous Substances Data) | ||
| 독성 | LC50 inhal (rat) 2500 ppm (1 h) PEL (OSHA) 2 ppm (5 mg/m3) TLV-TWA (ACGIH) 2 ppm (5.2 mg/m3) STEL (ACGIH) 4 ppm (10 mg/m3) |
||
| IDLA | 25 ppm | ||
| 기존화학 물질 | KE-25911 | ||
| 유해화학물질 필터링 | 97-1-246 | ||
| 사고대비 물질 필터링 | 46 | ||
| 함량 및 규제정보 | 물질구분: 유독물질; 혼합물(제품)함량정보: 질산 및 이를 10% 이상 함유한 혼합물 |
질산 C화학적 특성, 용도, 생산
물성
• 무색액체, 에테르/알코올에 가용(폭발성); • 황색으로 특이한 냄새를 갖는 부식성이 있는 액체로 공기에 접하면 자극성의 흰 연기를 내며 -40°C로 냉각시키면 응결 결정함; • 금, 백금을 제외한 거의 모든 금속을 부식시키며 질산염을 석출함; • 햇빛에 장시간 노출시 갈변현상.용도
유기합성, 니트로 화합물, 셀롤로이드공업, 화약, 폭약, 염료, 향료, 야금, 인견, 질산염 제조, 황산, 전기도금, 금속용해용, 사진제판, 의약품, 시약, 비료, TDI, 아디핀산, 표면처리제, 질산암모늄 등의 비료 제조, 로켓연료의 산화제 등개요
Nitric acid is a colorless, corrosive liquid that is the most common nitrogen acid. It has been used for hundreds of years. Nitric acid is a mineral acid that was called spirit of nitre and aqua fortis, which means strong water.
Fuming nitric acid is named because of the fumes emitted by acid when it combines with moist air. Fuming nitric acid is highly concentrated and is labeled either red fuming nitric acid or white fuming nitric acid. Red fuming nitric acid, as the name implies, emits a reddishbrown fume on exposure to air. The color comes from nitrogen dioxide, which is liberated on exposure to air. The nitric acid concentration of red fuming nitric acid is approximately 85% or greater, with a substantial amount of dissolved nitrogen dioxide. White fuming nitric acid is highly concentrated anhydrous nitric acid with concentrations of 98–99%; the remaining 1–2% is water and nitrogen dioxide. Most commercial grade nitric acid has a concentration of between 50% and 70%.
화학적 성질
Nitricacid,HN03, is a strong,fire-hazardous oxidant. It is a colorless or yellowish liquid that is miscible with water and boils at 86℃ (187 ℉). Nitric acid, also known as aqua fortis, is used for chemical synthesis, explosives, and fertilizer manufacture, and in metallurgy, etching, engraving, and ore flotation.물리적 성질
Colorless liquid; highly corrosive; refractive index 1.397 at 16.5°C; density 1.503 g/L; freezes at –42°C; boils at 83°C; completely miscible with water; forms a constant boiling azeotrope with water at 68.8 wt% nitric acid; the azeotrope has density 1.41 g/mL and boils at 121°C.용도
Nitric acid is an important starting material for the production of fertilizers and chemicals. Diluted nitric acid is used for dissolving and etching metals Product Data Sheet정의
nitric acid: A colourless corrosivepoisonous liquid, HNO3; r.d. 1.50;m.p. –42°C; b.p. 83°C. Nitric acid maybe prepared in the laboratory by thedistillation of a mixture of an alkalimetalnitrate and concentratedsulphuric acid. The industrial productionis by the oxidation of ammoniato nitrogen monoxide, theoxidation of this to nitrogen dioxide,and the reaction of nitrogen dioxidewith water to form nitric acid and nitrogenmonoxide (which is recycled).The first reaction (NH3 to NO) iscatalysed by platinum or platinum/rhodium in the form of fine wiregauze. The oxidation of NO and theabsorption of NO2 to form the productare noncatalytic and proceedwith high yields but both reactionsare second-order and slow. Increasesin pressure reduce the selectivity ofthe reaction and therefore ratherlarge gas absorption towers are required.In practice the absorbing acidis refrigerated to around 2°C and acommercial ‘concentrated nitric acid’at about 67% is produced.Nitric acid is a strong acid (highlydissociated in aqueous solution) anddilute solutions behave much likeother mineral acids. Concentrated niniobium tric acid is a strong oxidizing agent.Most metals dissolve to form nitratesbut with the evolution of nitrogenoxides. Concentrated nitric acid alsoreacts with several nonmetals to givethe oxo acid or oxide. Nitric acid isgenerally stored in dark brown bottlesbecause of the photolytic decompositionto dinitrogen tetroxide. Seealso nitration.
일반 설명
Nitric acid is a colorless to yellow or red liquid sometimes fuming reddish brown vapors with a suffocating odor. Nitric acid is soluble in water with release of heat. Nitric acid is corrosive to metals or tissue. Nitric acid will accelerate the burning of combustible materials and Nitric acid may even cause ignition upon contact with combustible material. Nitric acid is fully soluble in water and may react violently upon contact with water with the evolution of heat, fumes and spattering. Prolonged exposure to low concentrations or short term exposure to high concentrations may result in adverse health effects. Density 10.4 lb / gal.공기와 물의 반응
Fumes in air. Fully soluble in water with release of heat. Reacts violently with water with the production of heat, fumes, and spattering.반응 프로필
Nitric acid; ignites upon contact with alcohols, amines, ammonia, beryllium alkyls, boranes, dicyanogen, hydrazines, hydrocarbons, hydrogen, nitroalkanes, powdered metals, silanes, or thiols [Bretherick 1979. p.174]. The reaction of finely divided antimony and nitric acid can be violent [Pascal 10:504. 1931-34]. Bromine pentafluoride reacts violently with strong nitric acid and strong sulfuric acid [Mellor 2, Supp. 1:172. 1956]. Experiments show that mixtures of over 50% nitric acid by weight in acetic anhydride may act as detonating explosives [BCISC 42:2. 1971]. An etching agent of equal portions of acetone, nitric acid, and 75% acetic acid exploded 4 hours after Nitric acid was prepared and placed in a closed bottle. This is similar to a formulation for the preparation of tetranitromethane a sensitive explosive [Chem. Eng. News 38: 56. 1960]. Phosphine is violently decomposed by concentrated nitric acid, and flame is produced. Warm fuming nitric acid, dropped in a container of phosphine gas produces an explosion [Edin. Roy. Soc. 13:88. 1835]. An explosion occurs when nitric acid is brought into contact with phosphorus trichloride [Comp. Rend. 28:86]. The reaction of sodium azide and strong nitric acid is energetic [Mellor 8, Supp 2:315. 1967]. Reacts violently with water with the production of heat, fumes, and spattering.위험도
Because it is a strong oxidizing agent, nitric acid may undergo violent reactions with powerful reducing agents. Many nitration reactions of organics yield explosive products. Pure nitric acid is highly corrosive to skin causing severe injury. Concentrated acid (68.8 wt %) is moderately corrosive to skin. The acid may decompose under heating or photochemically, liberating toxic nitrogen dioxide gas.건강위험
Nitric acid is a corrosive substance causingyellow burns on the skin. It corrodes the bodytissues by converting the complex proteinsto a yellow substance called xanthoproteicacid (Meyer 1989). Ingestion of acid canproduce burning and corrosion of the mouthand stomach. A dose of 5–10 mL can befatal to humans.Chronic exposure to the vapor and mist ofnitric acid may produce bronchitis and chemical pneumonitis (Fairhall 1957). It emitsNO2, a highly toxic gas formed by its decomposition in the presence of light. Nitric acidis less corrosive than sulfuric acid. Its vaporand mist may erode teeth..
화재위험
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Vapors may accumulate in confined areas (basement, tanks, hopper/tank cars etc.). Substance will react with water (some violently), releasing corrosive and/or toxic gases and runoff. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water.인화성 및 폭발성
Explosibility Not a combustible substance, but a strong oxidizer. Contact with easily oxidizible materials including many organic substances may result in fires or explosions.공업 용도
Also called aqua fortis and azotic acid, nitricacid is a colorless to reddish fuming liquid ofthe composition HNO3, having a wide varietyof uses for pickling metals, etching, and in themanufacture of nitrocellulose, plastics, dyestuffs,and explosives. It has a specific gravityof 1.502 (95% acid) and a boiling point of 86°C,and is soluble in water. Its fumes have a suffocatingaction, and it is highly corrosive andcaustic. Fuming nitric acid is any water solutioncontaining more than 86% acid and having aspecific gravity above 1.480. Nitric acid is madeby the action of sulfuric acid on sodium nitrateand condensation of the fumes. It is also madefrom ammonia by catalytic oxidation, or fromthe nitric oxide produced from air.Safety Profile
Poison by inhalation. A corrosive irritant to skin, eyes, and mucous membranes. A very dangerous fire hazard and very powerful oxidizing agent. Can react explosively with many reducing agents. Wdl react with water or steam to produce heat and toxic, corrosive, and flammable vapors.When heated to decomposition it emits hghly toxic fumes of NOx. See also NITRIC ACID.Safety
Nitric acid is used in the manufacture of ammonium nitrate fertilizer and explosives, in steel etching, and in reprocessing spent nuclear fuel. There are two types of fuming nitric acid. White fuming nitric acid is concentrated with 97.5% nitric acid and less than 2% water. It is a colorless to pale-yellow liquid that fumes strongly. It is decomposed by heat and exposure to light and becomes red in color from nitrogen dioxide. Red fuming nitric acid contains more than 85% nitric acid, 6%–15% nitrogen dioxide, and 5% water. The four-digit UN identification number for red fuming nitric acid is 2032. The NFPA 704 designation is health 4, flammability 0, and reactivity 1. The prefix “oxy” appears in the white section of the diamond. Red fuming nitric acid is considered an oxidizer. Both white and red fuming acids are toxic by inhalation, strong corrosives, and dangerous fire risks that may explode upon contact with reducing agents. They are used in the production of nitro compounds, rocket fuels, and as laboratory reagents.잠재적 노출
Nitric acid is the second most important industrial acid and its production represents the sixth largest chemical industry in the United States. Nitric acid is used in chemicals, explosives, fertilizers, steel pickling; metal cleaning. The largest use of nitric acid is in the production of fertilizers. Almost 15% of the production goes into the manufacture of explosives, with the remaining 10% distributed among a variety of uses, such as etching, bright-dipping; electroplating, photoengraving, production of rocket fuel; and pesticide manufacture.Carcinogenicity
Nitric acid was not mutagenic in limited studies.4 There is no information regarding the carcinogenic properties of nitric acid, but an association between incidences of laryngeal cancer and exposure to acid mists has been indicated.4저장
Splash goggles and rubber gloves should be worn when handling this acid, and containers of nitric acid should be stored in a well ventilated location separated from organic substances and other combustible materials.운송 방법
UN2031 Nitric acid other than red fuming, with .70% nitric acid or Nitric acid other than red fuming, with at least 65%, but not >70% nitric acid, Hazard Class: 8; Labels: 8-Corrosive material, 5.1-Oxidizer. UN2032 Nitric acid, red fuming, Hazard Class: 8; Labels: 8-Corrosive material, 5.1-Oxidizer, 6.1-Poisonous material. Inhalation, Hazard Zone B. UN2031 Nitric acid other than red fuming, with >20% and <65% nitric acid or Nitric acid other than red fuming, with not >20% nitric acid, Hazard Class: 8; Labels: 8-Corrosive material.Purification Methods
The acid is obtained colourless (approx. 92%) by direct distillation of fuming HNO3 under reduced pressure at 40-50o with an air leak at the head of the fractionating column. Store it in a desiccator kept in a refrigerator. Nitrite-free HNO3 can be obtained by vacuum distillation from urea. [Ward et al. Inorg Synth III 13 1950, Kaplan & Schechter Inorg Synth IV 53 1953.]비 호환성
A strong oxidizer and strong acid. Reacts violently with combustible and reducing agents; carbides, hydrogen sulfide, turpentine, charcoal, alcohol, powdered metals; strong bases. Heat causes decomposition producing nitrogen oxides. Attacks some plastics. Corrosive to metals.폐기물 처리
Soda ash-slaked lime is added to form the neutral solution of nitrate of sodium and calcium. This solution can be discharged after dilution with water. Also, nitric acid can be recovered and reused in some cases as with acrylic fiber spin solutions. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.질산 준비 용품 및 원자재
원자재
백금분
질산마그네슘
일산화탄소(가스)
Compressor
cellular membranes
Electronic grade nitric acid
Air blower
산소, 냉각된 액체
질소 테트라산화물
산화 질소
Industrial nitric acid
Dehydrolyzing agent
Concentrated sulfuric acid
Heat exchanger
산화마그네슘
질산나트륨
암모니아(가스)
준비 용품
납 보레이트
PYRIDINE-2-SULFONIC ACID
3,5-DINITRO-4-HYDROXYBENZALDEHYDE
2-NITROTHIOPHENE-4-CARBOXALDEHYDE
1,3-Dimethyl-8-nitro-1H-purine-2,6(3H,9H)-dione ,97%
질산란탄
5-Amino-2,4,6-triiodo-N-methylisophthalamic Acid
질산카드뮴 사수화물
5-Methylisoxazole-3-carboxylic acid
2-NITROMESITYLENE
FLAVIANIC ACID
2-METHYL-5-NITRO-PYRIMIDINE-4,6-DIOL
5-Chloro-2-hydroxy-3-nitropyridine
네슬러 용액
3-Nitrobenzonitrile
2-Nitrothiophene
4-Acetamido-3-nitrobenzoic acid
6-NITROPIPERONAL
염화 수은(사이클로산)
페닐수은 질산
10-Nitroanthrone
Sodium nitrohumate
2,6-Dimethyl-3-nitropyridine
머큐로스 니트레이트
2-Hydroxy-5-nitronicotinic acid
5-NITROBARBITURIC ACID
3-METHYLPYRAZINE-2-CARBOXYLIC ACID
2-히드록시-3,5-디니트로피리딘
Mercury iodide
4,5-디페닐이미다졸
2-ethyl-5-nitrobenzenamine
3,3′-디니트로벤조페논
Mesaconic acid
mercurous bromide
Diethyl nitromalonate
질산 카드뮴
2,4-Dichloro-5-nitrobenzalehyde
hydrofining catalysts (RN series)
테트라 니트로메탄
Methyl 3-nitrobenzoate