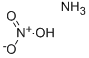질산암모늄
|
|
질산암모늄 속성
- 녹는점
- 169 °C(lit.)
- 끓는 점
- 210 °C(lit.)
- 밀도
- 1.72
- 인화점
- 210°C
- 저장 조건
- Store at RT.
- 용해도
- H2O: 1 M at 20 °C, 투명, 무색
- 물리적 상태
- 고체
- 물리적 상태
- 단단한 모양
- Specific Gravity
- 1.725
- 색상
- 하얀색
- 수소이온지수(pH)
- 4.5-6.0 (25℃, 50mg/mL in H2O)
- 수용성
- 190g/100mL(20℃)
- Merck
- 14,534
- CAS 데이터베이스
- 6484-52-2(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | O,Xi | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 8-36/37/38-9 | ||
| 안전지침서 | 17-26-36-37/39-41-16-15 | ||
| 유엔번호(UN No.) | UN 1942 5.1/PG 3 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | BR9050000 | ||
| F 고인화성물질 | 3-10 | ||
| TSCA | Yes | ||
| 위험 등급 | 5.1 | ||
| 포장분류 | III | ||
| HS 번호 | 31023090 | ||
| 유해 물질 데이터 | 6484-52-2(Hazardous Substances Data) | ||
| 독성 | LD50 orally in Rabbit: 2462 mg/kg | ||
| 기존화학 물질 | KE-01715 | ||
| 사고대비 물질 필터링 | 59 |
질산암모늄 C화학적 특성, 용도, 생산
개요
Ammonium nitrate is an oxidizer, which may explode under confinement and high temperatures. When mixed with fuel oil, a deflagrating explosive material is created. Ammonium nitrate and fuel oil were used as the explosive in the Alfred P. Murrah Federal Building terrorist attack in Oklahoma City and the first terror attack on the World Trade Center in New York City in the mid-1990s.화학적 성질
Ammonium nitrate,NH4N03, is a colorless crystalline solid existing in two forms, Between 16 and 32°C, the crystals are tetragonal; between 32 and 84 DC, the crystals are rhombic. The melting point of NH4N03 is 169.6 DC, and it decomposes above 210°C. When heated, ammonium nitrate yields nitrous oxide gas. Ammonium nitrate is soluble in water, in acetic acid solutions containing ammonia, is slightly soluble in ethanol, and is moderately soluble in methanol.
Ammonium nitrate is a very insensitive and stable high explosive used as a slow-burning propellant for rockets when compounded with burning rate catalysts. Although the major applications of Ammonium nitrate are explosives and fertilizers, it is also used in insecticides, rust inhibitors, and pyrotechnics.
용도
Ammonium nitrate (NH4NO3, also known as “Norway saltpeter”) is mainly used as a fertilizer. It is also known as the chemical that was mixed with diesel fuel to create the explosion that demolished the Murrah Federal Building in Oklahoma City in 1995.정의
ammonium nitrate: A colourlesscrystalline solid, NH4NO3; r.d. 1.72;m.p. 169.6°C; b.p. 210°C. It is verysoluble in water and soluble inethanol. The crystals are rhombicwhen obtained below 32°C andmonoclinic above 32°C. It may bereadily prepared in the laboratory bythe reaction of nitric acid with aqueousammonia. Industrially, it is manufacturedby the same reaction usingammonia gas. Vast quantities of ammoniumnitrate are used as fertilizers(over 20 million tonnes per year)and it is also a component of someexplosives.일반 설명
A colorless crystalline solid. Soluble in water. Does not readily burn but will do so if contaminated with combustible material. Accelerates the burning of combustible material. Produces toxic oxides of nitrogen during combustion. Used to make fertilizers and explosives, and as a nutrient in producing antibiotics and yeast.공기와 물의 반응
Water soluble. Hot aqueous solutions of the nitrate above 50% conc., under confinement may decompose explosively. This process is aided catalytically with such substances as nitric acid and chloride ion, [Chem. Abs., 1982, 97, 78074].반응 프로필
The hazards of AMMONIUM NITRATE have been well studied because of several extremely severe explosions [Chem. Eng., 1962, 70, 91; Bretherick, 5th Ed., 1995]. Mixtures with alkyl esters may explode, owing to the formation of alkyl nitrates. Mixtures with phosphorus, tin(II) chloride or other reducing agents may react explosively [Bretherick 1979 p. 108-109]. A mixture with aluminum powder (also zinc, cadmium, copper, magnesium, lead, cobalt, nickel, bismuth, chromium, and antimony) can be used as an explosive. A number of explosions in which ammonium nitrate and aluminum were mixed with carbon or hydrocarbons, with or without oxidizing agents have occurred [Mellor 5:219 1946-47]. A mixture with acetic acid ignites when warmed, especially if concentrated [Von Schwartz p. 322 1918]. Causes the decomposition of sodium hypochlorite within a few seconds [Mellor 2 Supp. 1:550 1956].위험도
May explode under confinement and high temperatures, but not readily detonated. Ventilate well. To fight fire, use large amounts of water. The material must be kept as cool as possible and removed from confinement and flooded with water in event of fire.건강위험
Inhalation, ingestion or contact (skin, eyes) with vapors or substance may cause severe injury, burns or death. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may cause pollution.화재위험
These substances will accelerate burning when involved in a fire. Some may decompose explosively when heated or involved in a fire. May explode from heat or contamination. Some will react explosively with hydrocarbons (fuels). May ignite combustibles (wood, paper, oil, clothing, etc.). Containers may explode when heated. Runoff may create fire or explosion hazard.잠재적 노출
Used in the manufacture of liquid and solid fertilizer compositions, industrial explosives and blasting agents from ammonium nitrate, matches; antibiotics; in the production of nitrous oxide.신진 대사
Ammonium Nitrate. Ammonium nitrate is one of the two leading nitrogen fertilizer materials on a world basis: 10% in 1997. The high N content is advantageous for the reduction of freight and application costs per unit weight of nitrogen. The presence of 50% of the nitrogen in the highly available nitrate form makes it suitable for use in regions growing crops with a short vegetation period but has the disadvantage that, because the NO3 ? ion is not adsorbed by soil, it may contribute to relatively large nitrogen losses by the leaching of increased soil nitrate into streams and groundwater. Although the application of any nitrogenous fertilizer results in some degree of soil acidification, the nitrate form is notably less acidifying than ammonium sulfate and has a lower tendency for the loss of nitrogen to the atmosphere as gaseous ammonia. The hygroscopic character of the crystalline material, coupled with its explosive nature, contributes to difficult storage and handling properties and the need for the production of purified and stabilized forms.운송 방법
Ammonium nitrate with organic coating: UN0222 Ammonium nitrate, with . 0.2% combustible substances, including any organic substance calculated as carbon, to the exclusion of any other added substance, Hazard Class: 1D; Labels:1D-Explosive (with a mass explosion hazard); D-Substances or articles which may mass detonate (with blast and/or fragment hazard) when exposed to fire. Ammonium nitrate with NO organic coating: UN1942 Ammonium nitrate, with NOT . 0.2% of combustible substances, including any organic substance calculated as carbon, to the exclusion of any other added substance (also used for fertilizer), Hazard Class: 5.1; Labels: 5.1-Oxidizer. UN3375 Ammonium nitrate emulsion or Ammonium nitrate suspension or Ammonium nitrate gel, intermediate for blasting explosives, Hazard Class: 5.1; Labels: 5.1-Oxidizer. UN2072 Ammonium nitrate fertilizer, n.o.s., doesn’t appear in the 49 CFR Hazmat Table, refer to UN1942, above). UN2071 Ammonium nitrate based fertilizer, Hazard class: 9; Labels: 9-Miscellaneous hazardous material. UN2426/140 Ammonium nitrate, liquid (hot concentrated solution), Hazard Class: 5.1; Labels: 5.1-Oxidizer.Purification Methods
It is crystallised twice from distilled water (1mL/g) by adding EtOH, or from warm water (0.5mL/g) by cooling in an ice-salt bath. Dry it in air, then under vacuum. After 3 recrystallisations of ACS grade, it contained Li and B at 0.03 and 0.74 ppm, respectively. It is deliquescent. [Early & Lowry J Chem Soc 115 1387 1919, 121 963 1922, Hendricks et al. J Am Chem Soc 54 2766 1932.]비 호환성
A strong oxidizer. Reducing agents; combustible materials; organic materials; finely divided (powdered) metals may form explosive mixtures or cause fire and explosions. When contaminated with oil, charcoal or flammable liquids, can be considered an explosive which can be detonated by combustion or shock.폐기물 처리
Pretreatment involves addition of sodium hydroxide to liberate ammonia and form the soluble sodium salt. The liberated ammonia can be recovered and sold. After dilution to the permitted provisional limit, the sodium salt can be discharged into a stream or sewer.질산암모늄 준비 용품 및 원자재
원자재
준비 용품
Compound fertilizer
황산코발트
염화니켈 헥사히드레이트
2,6-Diaminoanthraquinone
ammonium nitrate for technical
6-Aminobenzothiazole
노르플록사신
벤조티아졸
질산 바륨
Nitrogen phosphorus potassium mixed fertilizer
삼폴리인산 나트륨
설포메튜론-메틸
암모늄 폴리인산염
과산화 수소
Mixed and compound fertilizer
칼슘 리그노설포네이트
Lincomycin hydrochloride monohydrate
coating adhensive SSS-85
3-nitro-4-piperazin-1-ylbenzonitrile
4-BROMO-3-NITROBENZONITRILE
트리폴리인산나트륨
염화 암모늄
1-BOC-4-(4-CARBOXY-2-NITROPHENYL)PIPERAZINE
아산화질소
염산싸이아민
암모늄 바나딘산염
3-Amino-4-methoxybenzoic acid
D-Cycloserine
구아니딘 나이트레이트
리그노술폰산 나트륨
로듐
질산칼륨