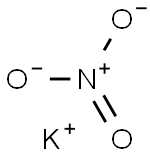
질산칼륨
|
|
질산칼륨 속성
- 녹는점
- 334 °C (lit.)
- 끓는 점
- 100 °C750 mm Hg
- 밀도
- 1.00 g/mL at 20 °C
- 벌크 밀도
- 800kg/m3
- 인화점
- 400°C
- 저장 조건
- Store at RT.
- 용해도
- H2O: 1 M at 20 °C, 투명, 무색
- 물리적 상태
- 고체
- 물리적 상태
- 단단한 모양
- Specific Gravity
- 2.109
- 색상
- 하얀색
- 냄새
- 무취, 시원한 매운맛, 짠맛
- 수소이온지수(pH)
- 5.0-7.5 (50g/l, H2O, 20℃)
- Flame Color
- Light Purple
- 수용성
- 320 g/L (20 ºC)
- 감도
- Hygroscopic
- Merck
- 14,7648
- Dielectric constant
- 5(0.0℃)
- 안정성
- 안정적인. 강산화제 - 가연성 물질과 접촉하면 화재가 발생할 수 있습니다. 가연성 물질, 강력한 환원제와 혼합할 수 없습니다.
- LogP
- -0.129 (est)
- CAS 데이터베이스
- 7757-79-1(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | O,Xi,Xn | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 8-36/38-36/37/38-22 | ||
| 안전지침서 | 26-17-36-7-24/25 | ||
| 유엔번호(UN No.) | UN 3264 8/PG 3 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | TT3700000 | ||
| TSCA | Yes | ||
| HS 번호 | 2834 21 00 | ||
| 위험 등급 | 5.1 | ||
| 포장분류 | III | ||
| 유해 물질 데이터 | 7757-79-1(Hazardous Substances Data) | ||
| 독성 | LD50 orally in rabbits: 1.166 g anion/kg, Dollahite, Rowe, Southwest. Vet. 27, 246 (1974) | ||
| 기존화학 물질 | KE-29163 | ||
| 사고대비 물질 필터링 | 63 |
질산칼륨 C화학적 특성, 용도, 생산
용도
1) 염화칼륨의 뜨거운 수용액에 질산나트륨(칠레 초석)을 첨가하여 복분해에 의해 생긴 염화나트륨의 결정을 분리하고 용액을 냉각하면 질산칼륨이 석출된다. 수용액에서 재결정에 의해 정제한다.2) 탄산칼륨 또는 수산화칼륨을 질산에 용해하여 제조한다.
용도
1) 흑색 화약, 성냥, 불꽃 등의 화공품의 제조.2) 유리, 유약의 원료, 유리의 청정제(특히 아비산과 함께 사용하여 그 청정 작용을 조장한다).
3) 열처리제(매용제라고도 한다).
4) 한제 및 육류의 색깔 고정제.
5) 산화제.
6) 의약(6국 기재. 이뇨, 발한, 소염약. 용량 : 1회 0.3g, 1일 1g 내복).
7) 분석 시약.
순도시험
(1) 용상 : 이 품목 1g을 물 10mL에 녹일 때, 그 액은 무색으로서 징명하여야 한다.
(2) 염화물 : 이 품목 0.5g을 취하여 염화물시험법에 따라 시험할 때, 그 양은 0.01N 염산 0.3mL에 대응하는 양 이하이어야 한다.
(3) 비소 : 이 품목을 비소시험법에 따라 시험할 때, 그 양은 4.0ppm 이하이어야 한다.
(4) 납 : 「메타인산나트륨」의 순도시험 (2)에 따라 시험한다(2.0ppm 이하).
(5) 수은 : 이 품목을 수은시험법에 따라 시험할 때, 그 양은 1.0ppm 이하이어야 한다.
(6) 아질산염 : 이 품목 1g을 정밀히 달아 「질산나트륨」의 순도시험 (5)에 따라 시험한다(20ppm 이하).
확인시험
이 품목은 확인시험법 중 칼륨염 및 질산염의 반응을 나타낸다.
정량법
이 품목을 건조한 다음 약 0.4g을 정밀히 달아 500mL 환저플라스크에 넣고 물 약 300mL에 녹여 데바르다합금분말 3g 및 수산화나트륨용액(2→5) 15mL를 가하여 비말방지기와 냉각기를 붙이고 받는 그릇에 0.1N 황산 50mL를 넣어 2시간 방치한 다음 유액 약 250mL를 얻을 때까지 증류하여 받는 그릇중의 과잉의 산을 0.1N 수산화나트륨용액으로 적정한다(지시약 : 메틸레드․메틸렌블루혼합시액 3방울). 따로 같은 방법으로 공시험을 한다.
0.1N 황산 1mL = 10.11mg KNO3
개요
Potassium nitrate is a solid, colorless, crystalline ionic compound that exists as the mineral niter.Potassium nitrate is also known as saltpeter. The name saltpeter comes from the Latin sal petrae, meaning salt of stone or salt of Petra. he term saltpeter or Chilean saltpeter is also used for sodium nitrate, NaNO3.화학적 성질
Potassium nitrate is an odorless, flammable, water-soluble, white or colorless crystals with saline taste that melt at 337°C. Used in pyrotechnics, explosives, and matches, as a fertilizer, and as an analytical reagent.물리적 성질
Colorless transparent crystals or white granular or crystalline powder;rhombohedral structure; density 2.11 g/cm3at 20°C; melts at 334°C; decomposes at 400°C evolving oxygen; soluble in cold water, 13.3 g/100mL at 0°C;highly soluble in boiling water, 247 g/100mL at 100°C; lowers the temperature of water on dissolution; very slightly soluble in ethanol; soluble in glycerol and liquid ammonia.역사
Saltpeter’s most prominent use in human history is as the principal ingredient in gunpowder.The potassium nitrate used in gunpowder was originally obtained from natural mineral deposits of niter. Small quantities formed as efflorescence deposits on damp stone walls were identified as early as 2000 b.c.e. in Sumerian writings. As the use of black powder expanded with the development of weapons, the demand for saltpeter exceeded supply. This was exacerbated during times of war. To meet the demand for saltpeter to produce black powder, a saltpeter industry developed that followed prescribed methods to produce large quantities of saltpeter. The method depended on processing dirt obtained from areas where nitrates would naturally form. These were areas in which animal waste had accumulated such as the dirt floors of barns, stables, herding pens, caves, or cellars. The ammonia compounds in the urine and fecal wastes in these areas underwent nitrifi cation to produce nitrates, which combined with potassium in the soil to form saltpeter.용도
Potassium Nitrate is a preservative and color fixative in meats which exists as colorless prisms or white granules or powder. it has a solubility of 1 g in 3 ml of water at 25°c. see nitrate.정의
ChEBI: The inorganic nitrate salt of potassium.생산 방법
Potassium nitrate may be produced by several methods. It is made commercially by reacting potassium chloride with nitric acid at high temperature.Nitrosyl chloride, a product obtained in the reaction, is converted into chlorine in this manufacturing process. Also, nitric acid is partly recycled in the process. The reactions are (Dancy, W.B. 1981. Potassium Compounds. In Kirk-Othmer Encyclopedia of Chemical Technology, 3rd. ed. Pp. 939-42. New York: Wiley Interscience):3KCl + 4HNO3 →3KNO3+ Cl2+ NOCl + 2H2O
2NOCl + 4HNO3→6NO2+ Cl2+ 2H2O
4NO2+ O2+ 2H2O →4HNO3
Potassium nitrate also can be prepared by mixing a hot saturated solution of potassium chloride and sodium nitrate. The reaction is:
K++ Clˉ+ Na++ NO3ˉ→NaCl↓+ K++ NO3ˉ
Sodium chloride is less soluble than KCl, NaNo3and KNo3. It separates out by crystallization. The remaining solution is cooled to ambient tempera-ture. Potassium nitrate crystallizes out.
World Health Organization (WHO)
Potassium nitrate was formerly used as a diuretic. Its use for this purpose is now considered obsolete but it is still available in at least one country for the correction of potassium deficiency. It is aslo widely permitted at concentrations of the order of 5% in proprietary toothpastes. In some countries the drug has been banned due to a potential carcinogenic risk arising from the excessive use of nitrates and their transformation to nitrosamines.일반 설명
A white to dirty gray crystalline solid. Water soluble. Noncombustible, but accelerates the burning of combustible materials. If large quantities are involved in fire or the combustible material is finely divided an explosion may result. May explode under prolonged exposure to heat or fire. Toxic oxides of nitrogen are produced in fires. Used in solid propellants, explosives, fertilizers.공기와 물의 반응
Soluble in water.반응 프로필
Potassium nitrate mixed with alkyl esters may explode, owing to the formation of alkyl nitrates; mixtures with phosphorus, tin (II) chloride, or other reducing agents may react explosively [Bretherick 1979. p. 108-109]. Powdered antimony mixed with Potassium nitrate explodes when heated [Mellor 9:282 1946-47]. A mixture of antimony trisulfide and Potassium nitrate explodes at a red heat [Mellor 9:524. 1946-47]. Arsenic disulfide forms explosive mixtures when mixed with Potassium nitrate, [Mellor 9:270.1946-47]. A mixture of sodium acetate and Potassium nitrate may cause an explosion [Pieters 1957. p. 30]. A mixture of Potassium nitrate and sodium hypophosphite constitutes a powerful explosive [Mellor 8:881. 1946-47]. A mixture of powdered zirconium and Potassium nitrate explodes when heated above the melting point [Mellor 7:116. 1946-47].위험도
Dangerous fire and explosion risk when shocked or heated, or in contact with organic mate- rials, strong oxidizing agent.건강위험
Exposure can cause mild irritation of eyes, nose and throat.농업용
Potassium nitrate (KNO3) is a potassium salt of nitric acid, also known as saltpeter or nitrate of potash. It is a white crystalline salt which occurs naturally in nitre or saltpeter. It can be used as fertilizer for normal application and fertigation. Potassium (44% K2O) and nitrogen (13 %) are the constituents of NK fertilizers, which serve as a source of potassium, where extra chloride is not desired.The agricultural grade of potassium nitrate is freeflowing and non-caking, with a particle size in the range of 1500 to 400 microns.
Potassium nitrate, which is slightly hygroscopic and granulated, can be spread on soil by trucks, fertilizer distributors or by aerial spraying. In a mixed fertilizer, a powdered grade of nitrate of potash does not cake. Potassium nitrate is made by the reaction of potassium chloride with nitric acid as: The nitrate of potash forms an easily breakable crust on top. It is chemically neutral and its nitrogen and potassium oxide ratio is roughly 1:3. It has been used successfully as a source of nitrogen and potassium for tobacco, tomato, potato, corn, citrus and carnations.
공업 용도
Potassium nitrate is also called niter and saltpeter,although these usually refer to the nativemineral. A substance of the composition KNO3,it is used in explosives, for bluing steel, and infertilizers. A mixture of potassium nitrate andsodium nitrate is used for steel-tempering baths.The mixture melts at 250°C. Potassium nitrateis made by the action of potassium chloride onsodium nitrate. It occurs in colorless prismaticcrystals, or as a crystalline white powder. It hasa sharp saline taste and is soluble in water. Thespecific gravity is 2.1 and the melting point is337°C.Potassium nitrate contains a large percentageof oxygen, which is readily given up andis well adapted for pyrotechnic compounds. Itgives a beautiful violet flame in burning. It isused in flares and in signal rockets.
Most enamels contain some oxidizing agentin the form of potassium or sodium nitrate.Only a small amount of nitrate is necessary; 2to 4% is sufficient to maintain oxidizing conditionsin most smelting operations.
In glazes it is sometimes used as a flux inplace of potassium oxide, but, owing to its costand solubility, very little of it is contained inglaze. Where conditions prevent the use of sufficientpotash feldspar, potassium oxide is introducedinto the mix, usually in the form of thenitrate in a frit.
Potassium nitrite is a solid of the compositionKNO2 used as a rust inhibitor, for theregeneration of heat-transfer salts, and for themanufacture of dyes.
Safety Profile
Poison by intravenous route. Moderately toxic by ingestion. An experimental teratogen. Experimental reproductive effects. Mutation data reported. Ingestion of large quantities may cause gastroenteritis. Chronic exposure can cause anemia, nephritis, and methemoglobinemia. When heated, reaction with calcium hydroxide + polychlorinated phenols forms extremely toxic chlorinated benzodtoxins. A powerful oxidizer. Gunpowder is a mixture of potassium nitrate + sulfur + charcoal. Explosive reaction with aluminum + barium nitrate + potassium perchlorate + water (in storage), boron + laminac + trichloroethylene. Forms explosive mixtures with lactose, powdered metals (e.g., titanium, antimony, germanium), metal sulfides (e.g., antimony trisulfide, barium sulfide, calcium sulfide, germanium monosulfide, titanium disulfide, arsenic disulfide, molybdenum disulfide), nonmetals (e.g., boron, carbon, white phosphorus, arsenic), organic materials, phosphides (e.g., copper(l1) phosphide, copper monophosphide), reducing agents (e.g., sodium phosphinate, sodium thiosulfate), sodium acetate. Can react violently under the appropriate conditions with 1,3- bis(trichlorometh~d)benzene, boron phosphde, F2, calcium shcide, charcoal, chromium nitride, Na hypophosphte, ma2O2 + dextrose), red phosphorus, (S + As2S3), thorium dicarbide, trichloroethylene, zinc, zirconium. When heated to decomposition it emits very toxic fumes of NOx and K2O. See also NITRATES.잠재적 노출
Used to make explosives, gunpowder, fireworks, rocket fuel; matches, fertilizer, fluxes, glass manufacture; and as a diuretic운송 방법
UN1486 Potassium nitrate, Hazard Class: 5.1; Labels: 5.1-Oxidizer.Purification Methods
It crystallises from hot H2O (0.5mL/g) on cooling (cf KNO2 below). Dry it for 12hours under vacuum at 70o. The solubility in H2O is 13.3% at 0o, 110% at 60o, and 246% at 100o. After two recrystallisations, technical grade salt had <0.001 ppm of metals. The fused salt is a powerful oxidising agent.비 호환성
A powerful oxidizer. Dangerously reactive and friction-and shock-sensitive when mixed with organic materials and many materials. Violent reactions with reducing agents; chemically active metals; charcoal, trichloroethylene.질산칼륨 준비 용품 및 원자재
원자재
준비 용품
1-브로모-4-(메틸설포닐)-2-니트로벤젠
Compound fertilizer
트리메틸퀴논
7-메틸라데닌
8-Aminoisoquinoline
4-NITROQUINOLINE N-OXIDE
Acifluorfen
ammonia synthesis Fe catalysts
1-플루오로-4-(트리플루오로메틸티오)벤젠
2,6-Dinitroaniline
4-METHYL-5-NITRO-2-피리딘카르복실산
Nitrogen phosphorus potassium mixed fertilizer
ISOPROPYLSULFONYL CHLORIDE
5-Nitro-6-methyluracil
겐타미신 황산염
7-Aminoisoquinoline
요오드화 은
3-Amino-4,6-dimethylpyridine
ethybenzene dehydrogenation catayst NCY
6-AMINO-2-N-BOC-1,2,3,4-TETRAHYDRO-ISOQUINOLINE
디미듐 브로마이드
알리자린
1-메틸-4-니트로-1H-이미다졸-5-카르보니트릴
8-Bromoisoquinoline
3-Amino-2-bromo-4,6-dimethylpyridine
AMMONIA SYNTHESIS CATALYST
5-클로로-1-메틸-4-니트로이미다졸
3-Amino-4-nitrobenzitrifluoride
6-니트로인돌
3-니트로벤즈알데히드
요오드(옥소)산 칼슘
4-AMINO-3-FORMYLPYRIDINE
과요드산 칼륨
6-아미노인돌
텅스텐산나트륨
아질산칼륨
4-Methyl-3-nitropyridine
4-비닐피리딘
7-니트로-3,4-디하이드로이소퀴놀린
2,4-DICHLORO-3-METHYL-5-NITROBENZOIC ACID
질산칼륨 관련 검색:
과황산 칼륨 아세트산 칼륨 황산칼륨 브로민산 칼륨 염화칼륨 칼륨 아크릴산 솔빈산칼륨 칼륨 질산 질산칼륨 사이안화 칼륨 아질산칼륨
Losartan potassium
POTASSIUM NITRITE (15N)
POTASSIUM NITRATE-15N
POTASSIUM NITRATE-15N-18O3, 99 ATOM % 15 N, 95 ATOM % 18O,POTASSIUM NITRATE-15N-18O3 98+ ATOM %
POTASSIUM, STANDARD SOLUTION 1000 MG/L K FOR ICP (POTASSIUM NITRATE IN WATER)
IAEAN3-POTASSIUM NITRATE