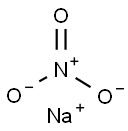
질산나트륨
|
|
질산나트륨 속성
- 녹는점
- 306 °C (dec.) (lit.)
- 끓는 점
- 380 °C
- 밀도
- 1.1 g/mL at 25 °C
- 저장 조건
- Store at RT.
- 용해도
- H2O: 1 M at 20 °C, 투명, 무색
- 물리적 상태
- 고체
- 물리적 상태
- 단단한 모양
- Specific Gravity
- 2.261
- 색상
- 흰색 또는 무색
- 수소이온지수(pH)
- 5.5-8.0 (50g/l, H2O, 20℃)
- 냄새
- 냄새 없는
- 수용성
- 900g/L(20℃)
- 감도
- Hygroscopic
- Merck
- 14,8647
- Dielectric constant
- 5.2(0.0℃)
- 안정성
- 안정적인. 강한 산화제 - 가연성 물질을 발화시킬 수 있습니다. 시안화물, 가연성 물질, 강력한 환원제, 알루미늄과 혼합되지 않습니다.
- LogP
- -0.129 (est)
- CAS 데이터베이스
- 7631-99-4(CAS DataBase Reference)
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | O,Xn,Xi,C | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 8-22-36/37/38-36/38-34-36 | ||
| 안전지침서 | 17-26-27-36/37/39-37/39-36-45 | ||
| 유엔번호(UN No.) | UN 1498 5.1/PG 3 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | WC5600000 | ||
| F 고인화성물질 | 3 | ||
| TSCA | Yes | ||
| 위험 등급 | 5.1 | ||
| 포장분류 | III | ||
| HS 번호 | 31025090 | ||
| 유해 물질 데이터 | 7631-99-4(Hazardous Substances Data) | ||
| 독성 | LD50 orally in rabbits: 1.955 g anion/kg (Dollahite, Rowe) | ||
| 기존화학 물질 | KE-31545 | ||
| 사고대비 물질 필터링 | 67 |
질산나트륨 C화학적 특성, 용도, 생산
순도시험
(1) 용상 : 「질산칼륨」의 순도시험 (1)에 따라 시험한다.
(2) 비소 : 이 품목을 비소시험법에 따라 시험할 때, 그 양은 4.0ppm 이하이어야 한다.
(3) 납 : 「메타인산나트륨」의 순도시험 (2)에 따라 시험한다(2.0ppm 이하).
(4) 수은 : 이 품목을 수은시험법에 따라 시험할 때, 그 양은 1.0ppm 이하이어야 한다.
(5) 아질산염 : 이 품목 1g을 정밀히 달아 물에 녹여 100mL로 한다. 이 액 20mL를 취하여 100mL 플라스크에 넣고 물을 가하여 약 80mL로 한 다음 설파닐아미드용액 10mL를 가해 주고 혼합한다. 3분후 커플링용액 1mL를 가해 주고 물을 가하여 100mL로 한 다음 잘 섞어 주고 15분 동안 방치한 액을 파장 540nm에서 흡광도를 측정한다. 검량선 및 다음 계산식에 따라 아질산염의 양을 구할 때, 그 양은 30ppm 이하이어야 한다.
A : 검량선으로부터 구한 아질산염의 양(μg)
W : 검체의 채취량(g)
검량선의 작성 : 아질산나트륨 0.75g을 정밀히 달아 물에 녹여 1,000mL로 하고 이 액 10mL에 물을 가하여 100mL로 하고, 다시 이 액 10mL에 물을 가하여 1,000mL로 한 액을 표준용액으로 한다. 각 표준용액 0, 5, 10, 20 및 50mL(각 액 1mL는 아질산염 0, 2.5, 5, 10 및 25μg 함유)를 취하여 각각의 100mL 플라스크에 넣고 물을 가하여 약 80mL씩으로 한 다음, 각 액에 설파닐아미드용액 10mL를 가해 주고 혼합한다. 3분 후 각각에 커플링용액 1mL씩을 가해 주고 물을 가하여 100mL씩으로 한 다음 잘 섞어 주고 15분 동안 방치한 다음 각 액을 파장 540nm에서 흡광도를 측정하여 검량선을 작성한다.
설파닐아미드용액 : 설파닐아미드 2g을 묽은염산에 녹여 1,000mL로 한다.
커플링용액 : N-1-나프틸에틸렌디아민이염산 0.2g을 물에 녹여 100mL로 한다.
(6) 염화물 : 이 품목 0.1g을 취하여 염화물시험법에 따라 시험할 때, 그 양은 0.01N 염산 0.6mL에 대응하는 양 이하이어야 한다.
확인시험
이 품목은 확인시험법 중 질산염 및 나트륨염의 반응을 나타낸다.
개요
Sodium nitrate, also known as Chile saltpeter and soda niter, has a molecular formula of NaNO3. Sodium nitrate is a colorless, odorless, transparent crystal. It oxidizes when exposed to air and is soluble in water. This material explodes at 1000°F (537°C), much lower than temperatures encountered in many fires. Sodium nitrate is toxic by ingestion and has caused cancer in test animals. When used in the curing of fish and meat products, it is restricted to 100 ppm. Sodium nitrate is incompatible with ammonium nitrate and other ammonium salts. The four-digit UN identification number is 1498. Sodium nitrate is used as an antidote for cyanide poisoning and in the curing of fish and meat.화학적 성질
Sodium nitrate, NaNO3, also known as soda niter and Chile saltpeter, is a fire-hazardous, transparent, colorless and odorless crystalline solid. It is soluble in glycerol and water,decomposes when heated,and melts at 308°C (585 °F). Sodium nitrate is used in making nitric and sulfuric acids, in the manufacture of glass and pottery enamel, as a fertilizer, as a food preservative, in explosives, and as a welding flux.물리적 성질
Colorless crystalline solid; saline taste; trigonal, and rhombohedrals structure; density 2.257g/cm3; refractive index 1.587 (trigonal) and 1.336 (rhombohedral); melts at 308°C; decomposes at 380°C; specific conductance 95 μmhos/cm at 300°C; viscosity 2.85 centipoise at 317°C; very soluble in water 92.1 g/100 mL at 25°C and 180 g/100 mL at 100°C; very soluble in liquid ammonia; soluble in alcohol.출처
There are several natural deposits of sodium nitrate in various parts of the world, including Chile, Mexico, Egypt, and the United States. The most important application of sodium nitrate is its use as a fertilizer in agriculture. It is an effective fertilizer for cotton, tobacco, and vegetable crops. Its agricultural applications, however, have dwindled considerably in recent years because of the growth of ammonium nitrate and other fertilizers.Another major use of sodium nitrate is in manufacturing explosives. It is a component of many types of dynamites and water-based slurry type blasting explosives. Sodium nitrate also is used in making charcoal briquettes. Sodium nitrate is used as an oxidizing and fluxing agent in manufacturing vitreous glass, fiberglass, porcelain, and enamels. Other uses are in the heat-treatment baths for alloys and metals, as a food preservative, in curing meats, and in preparing various salts.
용도
Sodium Nitrate is the salt of nitric acid that functions as an antimi- crobial agent and preservative. it is a naturally occurring substance in spinach, beets, broccoli, and other vegetables. it consists of color- less, odorless crystals or crystalline granules. it is moderately deli- quescent in moist air and is readily soluble in water. it is used in meat curing to develop and stabilize the pink color. see nitrate.생산 방법
Sodium nitrate is recovered from natural deposits. One such process, known as the Guggenheim nitrate process, is briefly outlined below: The ore is crushed. Sodium nitrate is leached from the ore by extraction with a brine solution at 40°C. The brine for leaching is made up of an aqueous solution of magnesium sulfate, MgSO3, and calcium sulfate, CaSO3. The caliche variety of Chilean ore contains mostly sodium nitrate and sodium chloride as the main saline components, along with limestone, clays, sand, lime, and inert volcanic rocks. Sodium nitrate usually occurs in this ore as a double salt with sodium sulfate NaNO3?Na2SO3?H3O. This double salt, which is sparingly soluble in water, is broken down by magnesium in leaching brine solution, thus releasing more sodium nitrate into the extract. Sodium nitrate finally is recovered from the leachate brine by fractional crystallization.Brines of other compositions have been used to extract sodium nitrate from its ores. Many such processes, including the Shanks process practiced in the past to produce sodium nitrate, are now obsolete.