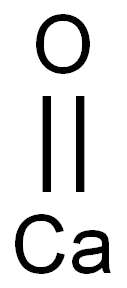산화칼슘(생석회)
|
|
산화칼슘(생석회) 속성
- 녹는점
- 2570 °C
- 끓는 점
- 2850 °C (lit.)
- 밀도
- 3.3 g/mL at 25 °C (lit.)
- 굴절률
- 1.83
- 인화점
- 2850°C
- 저장 조건
- no restrictions.
- 용해도
- 1.65g/l 격렬한 반응의 위험이 있습니다.
- 물리적 상태
- 가루
- 색상
- 흰색~노란색~아주 약간 베이지색
- Specific Gravity
- 3.3
- 수소이온지수(pH)
- 12.6 (H2O, 20℃)(saturated solution)
- 냄새
- 무취 백색 회색 결정 또는 분말
- 수용성
- 물과 반응
- 감도
- Air & Moisture Sensitive
- Crystal Structure
- Cubic
- Merck
- 14,1686
- Dielectric constant
- 2.2(Ambient)
- 노출 한도
- ACGIH: TWA 2 mg/m3
OSHA: TWA 5 mg/m3
NIOSH: IDLH 25 mg/m3; TWA 2 mg/m3
- 안정성
- 안정적이지만 공기 중 이산화탄소를 흡수합니다. 물, 습기, 불소, 강산과 호환되지 않습니다.
- InChIKey
- ODINCKMPIJJUCX-UHFFFAOYSA-N
- CAS 데이터베이스
- 1305-78-8(CAS DataBase Reference)
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | C,Xi | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 34-41-37/38 | ||
| 안전지침서 | 26-36/37/39-45-25-39 | ||
| 유엔번호(UN No.) | 1910 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | EW3100000 | ||
| F 고인화성물질 | 10-21-34 | ||
| TSCA | Yes | ||
| 위험 등급 | 8 | ||
| 포장분류 | III | ||
| HS 번호 | 28259019 | ||
| 유해 물질 데이터 | 1305-78-8(Hazardous Substances Data) | ||
| IDLA | 25 mg/m3 | ||
| 기존화학 물질 | KE-04588 |
산화칼슘(생석회) C화학적 특성, 용도, 생산
순도시험
(1) 산불용물 : 이 품목 5g에 물 100mL 및 충분한 염산을 방울로 가하여 용액이 되게 한다. 이 액을 끓이고 식힌 다음 필요하면 액을 뚜렷한 산이 되도록 염산을 가하고 미리 칭량한 도자기로 여과한다. 잔류물을 씻은 물이 염화물반응을 나타내지 않을 때까지 씻어내고 105℃에서 1시간 건조할 때, 그 양은 1.0% 이하이어야 한다.
(2) 알칼리 또는 마그네슘 : 이 품목 0.5g을 물 30mL 및 묽은염산 15mL에 녹이고 1분간 끓인 다음 즉시 수산시액 40mL를 가하여 격렬히 흔든다. 메틸레드시액 2방울을 가하고 칼슘을 완전히 침전시키기 위하여 암모니아시액으로 액을 중화시킨다. 이 액을 수욕상에서 1시간 동안 가열하고 식힌 다음 물을 가하여 100mL로 하고 여과한다. 여액 50mL에 황산 0.5mL를 가하여 증발건고한 다음 백금도가니를 800±25℃에서 항량이 될 때까지 강열할 때, 그 양은 3.6% 이하이어야 한다.
(3) 불소화물 : 이 품목 1g을 정밀히 달아 「구연산칼슘」의 순도시험 (8)에 따라 시험한다(50ppm 이하).
(4) 비소 : 이 품목을 비소시험법에 따라 시험할 때, 그 양은 4.0ppm 이하이어야 한다.
(5) 바륨 : 이 품목 1.5g을 달아 물 10mL와 혼합하고 묽은염산 15mL를 가한 다음 물로 30mL로 하고 여과한 액을 시험용액으로 한다. 따로, 바륨표준용액 0.3mL를 취하고 물을 가하여 20mL로 한 액을 대조액으로 한다. 시험용액 20mL 및 대조액에 초산나트륨 2g, 묽은초산 1mL를 및 크롬산칼륨시액 0.5mL를 가하고 15분간 방치할 때, 시험용액이 나타내는 탁도는 대조액의 탁도 이하이어야 한다(0.03% 이하).
(6) 납 : 「메타인산나트륨」의 순도시험 (2)에 따라 시험한다(2.0ppm 이하).
확인시험
이 품목 1g에 물 20mL를 가하고 녹을 때까지 초산을 가한 액은 확인시험법 중 칼슘염의 반응을 나타낸다.
정량법
이 품목 1g을 항량이 될 때까지 강열한 다음 정밀히 달아 묽은염산 20mL에 녹이고 식힌 다음 물을 가하여 500mL로 한다. 이 액 50mL를 취하여 물 50mL를 넣고 저으면서 수산화나트륨시액 15mL 및 히드록시나프톨블루(C20H12O11S3Na2) 0.3g을 가한 다음 0.05M 이.디.티.에이.용액으로 적정한다. 종말점은 적색이 완전히 소실되고 청색이 된 점으로 한다.
0.05M 이.디.티.에이.용액 1mL = 2.804mg CaO
화학적 성질
Calcium oxide, CaO, occurs as white or grayish-white lumps or granular powder. The presence of iron gives it a yellowish or brownish tint.물리적 성질
Calcium oxide is a white caustic crystalline alkali substance that goes by the common name lime. The term lime is used both generically for several calcium compounds and with adjectives to qualify different forms of lime. This entry equates lime, also called quicklime or burnt lime, with the compound calcium oxide. Hydrated lime, made by combining lime with water, is calcium hydroxide and is often referred to as slaked lime (Ca(OH)2). Dolomite limes contain magnesium as well as calcium. Limestone is the compound calcium carbonate. The term lime comes from the Old English word l?m for a sticky substance and denotes lime’s traditional use to produce mortar. Calx was the Latin word for lime and was used to name the element calcium.역사
Calcium oxide dates from prehistoric times. It is produced by heating limestone to drive off carbon dioxide in a process called calcination: CaCO3(s) CaO(s) + CO2(g). At temperatures of several hundred degrees Celsius, the reaction is reversible and calcium oxide will react with atmospheric carbon dioxide to produce calcium carbonate. Efficient calcium oxide production is favored at temperatures in excess of 1,000°C. In prehistoric times limestone was heated in open fires to produce lime. Over time, lined pits and kilns were used to produce lime. Brick lime kilns were extensively built starting in the 17th century and the technology to produce lime has remained relatively constant since then.용도
Calcium Oxide is a general food additive consisting of white granules or powder of poor water solubility. it is obtained by heating limestone (calcium carbonate) in a furnace. it is also termed lime or quicklime. it is used as an anticaking agent, firming agent, and nutritive supple- ment in applications such as grain products and soft candy.생산 방법
Calcium oxide is commercially obtained from limestone. The carbonate is roasted in a shaft or rotary kiln at temperatures below 1,200°C until all CO2 is driven off. The compound is obtained as either technical, refractory or agri cultural grade product. The commercial product usually contains 90 to 95% free CaO. The impurities are mostly calcium carbonate, magnesium carbon ate, magnesium oxide, iron oxide and aluminum oxide.정의
ChEBI: Calcium oxide is a member of the class of calcium oxides of calcium and oxygen in a 1:1 ratio. It has a role as a fertilizer.일반 설명
Calcium oxide appears as an odorless, white or gray-white solid in the form of hard lumps. A strong irritant to skin, eyes and mucous membranes. Used in insecticides and fertilizers.공기와 물의 반응
Crumbles on exposure to moist air. Reacts with water to form corrosive calcium hydroxide, with evolution of much heat. Temperatures as high as 800° C have been reached with addition of water (moisture in air or soil). The heat of this reaction has caused ignition of neighboring quantities of sulfur, gunpowder, wood, and straw [Mellor 3: 673 1946-47].반응 프로필
A base and an oxidizing agent. Neutralizes acids with generation of heat. Nonflammable, but will support combustion by liberation of oxygen, especially in the presence of organic materials. Reacts very violently with liquid hydrofluoric acid [Mellor 2, Supp. 1:129 1956]. Reacts extremely violently with phosphorus pentaoxide when reaction is initiated by local heating [Mellor 8 Supp.3:406 1971].위험도
Evolves heat on exposure to water. Danger- ous near organic materials. Upper respiratory tract irritant.건강위험
Causes burns on mucous membrane and skin. Inhalation of dust causes sneezing.화재위험
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Vapors may accumulate in confined areas (basement, tanks, hopper/tank cars etc.). Substance will react with water (some violently), releasing corrosive and/or toxic gases and runoff. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water.농업용
Calcium oxide (CaO) is a white powder with a neutralizing value or calcium carbonate equivalent (CCE) of 179%, compared to 100% for calcium carbonate (CaCO3). For quick results, either calcium oxide or calcium hydroxide [Ca(OH)2] is used. Calcium oxide is also known as lime, unslaked lime, burned lime or quicklime. Roasting CaCO3 in a furnace makes calcium oxide. A complete mixing of calcium oxide with soil is difficult because it cakes due to absorption of water.공업 용도
Lime is the most widely used reagent in the mineral industry for flotation of sulfides and, in some cases, non-sulfide minerals. The word “lime” is a general term used to describe any kind of calcareous material or finely divided form of limestone and dolomite. In more strict chemical terms, lime is calcinated limestone known as calcium oxide (CaO), quicklime or unslaked lime.The slaked or hydrated lime Ca(OH)2 is the form of lime primarily used in mineral flotation. Production of high-calcium lime is based on calcination of limestone at a temperature of 1100–1300 °C in kilns.CaCO3+heat--->CaO+CO2 For high-magnesium (dolomitic) limestone, the calcination reaction (at 1000–1200 °C) is CaCO3·MgCO3 (limestone) + heat--->CaOMgO (quicklime-2CO2)
Safety Profile
A caustic and irritating material. See also CALCIUM COMPOUNDS. A common air contaminant. A powerful caustic to living tissue. The powdered oxide may react explosively with water. Mixtures with ethanol may igmte if heated and thus can cause an air-vapor explosion. Violent reaction with (I3203 + CaCl2) interhalogens (e.g., BF3, CIF3), F2, HF, P2O5 + heat, water. Incandescent reaction with liquid HF. Incompatible with phosphoms(V) oxide.잠재적 노출
Calcium oxide is used as a refractory material; a binding agent in bricks; plaster, mortar, stucco, and other building materials. A dehydrating agent, a flux in steel manufacturing, and a labora운송 방법
UN1910 Calcium oxide, Hazard class: 8; Labels: 8-Corrosive material.비 호환성
The water solution is a medium strong base. Reacts with water, forming calcium hydroxide and sufficient heat to ignite nearby combustible materials. Reacts violently with acids, halogens, metals.폐기물 처리
Pretreatment involves neutralization with hydrochloric acid to yield calcium chloride. The calcium chloride formed is treated with soda ash to yield the insoluble calcium carbonate. The remaining brine solution may be discharged into sewers and waterways산화칼슘(생석회) 준비 용품 및 원자재
원자재
준비 용품
산화칼슘(생석회) 공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 |
sales@hbmojin.com | China | 12456 | 58 |
| Chemson Industrial (Shanghai) Co., Ltd. | 86-21-65208861- 8007 |
sales1@chemson.com.cn | CHINA | 117 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 |
alice@crovellbio.com | China | 8823 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 |
sales@chemdad.com | China | 39916 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 |
sales@conier.com | China | 49390 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-87569266 15319487004 |
1015@dideu.com | China | 2263 | 58 |
| Beijing CommScope Huiwei Technology Co., Ltd | 86-18810111057 |
summer@kphwchem.com | CHINA | 51 | 58 |
| SIMAGCHEM CORP | +86-13806087780 |
sale@simagchem.com | China | 17367 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 |
sales@tnjchem.com | China | 34572 | 58 |