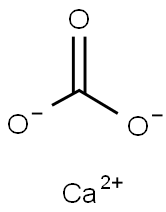
석회석
|
|
석회석 속성
- 녹는점
- 825 °C
- 끓는 점
- 2850 °C
- 밀도
- 2.93 g/mL at 25 °C(lit.)
- 용해도
- 5 M HCl: 20°C에서 0.1°M, 투명, 무색
- 물리적 상태
- 랜덤 크리스털
- 색상
- 회색
- Solubility Product Constant (Ksp)
- pKsp: 8.47
- CAS 데이터베이스
- 1317-65-3(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xi | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 37/38-41 | ||
| 안전지침서 | 26-36/37/39 | ||
| WGK 독일 | - | ||
| RTECS 번호 | FF9335000 | ||
| 기존화학 물질 | KE-21996 |
석회석 C화학적 특성, 용도, 생산
개요
Calcite is one of the most common minerals on the face of the earth, comprising about 4% by weight of the earth’s crust and is formed in many different geological environments. Calcite can form rocks of considerable mass and constitutes a significant part of all three major rock classification types. It forms oolitic, fossiliferous and massive limestones in sedimentary environments and even serves as the internal cement for many sandstones and shales.Calcite is even a major component in the igneous rock called carbonatite and forms the major portion of many hydrothermal veins. Not necessarily a variety of calcite, cave formations are certainly a unique aspect of calcite’s story.화학적 성질
Calcium carbonate is a white, odorless powder, or crystalline solid.용도
Source of lime; neutralizing agent, filler, and extender in rubber, plastics, paints; opacifying agent in paper; fortification of bread; putty; tooth pow- ders; antacid; whitewash; Portland cement; sulfur dioxide removal from stack gases; metallurgical flux; analytical chemistry; carbon dioxide gener- ation (laboratory).제조 방법
The calcite present is derived mostly from the remains of organisms such as clams, brachiopods, bryozoa, crinoids and corals. These animals live on the bottom of the sea and when they die their shells accumulate into piles of shelly debris. This debris can then form beds of limestone. Some limestones may have been derived from nonbiogenic calcite formation.정의
Aragonite: An anhydrous mineral form of calcium carbonate, CaCO3, which occurs associated with limestone and in some metamorphic rocks. It is also the main ingredient of pearls. It is not as stable as calcite, into which it may change over time.Calcite:A mineral form of calcium carbonate occurring in limestone, chalk, and marble.
Charlk: A natural form of calcium carbonate (CaCO3) formed originally by marine organisms. (Blackboard chalk is calcium sulfate, CaSO4.).
일반 설명
Odorless, white to tan powder.반응 프로필
CALCIUM CARBONATE has generally low chemical reactivity. Is non-combustible. Decomposes at high temperature (825°C) to give gaseous carbon dioxide and calcium oxide (quicklime). Incompatible with acids, alum, ammonium salts, fluorine, magnesium. Reacts with acids and acidic salts to generate gaseous carbon dioxide with effervescence (bubbling). The reaction is rapid and exothermic with concentrated solutions of acids. The efferversence can create extensive foaming. Ignites on contact with fluorine.위험도
A nuisance particulate dust.건강위험
Calcium carbonate is considered to be a nuisance dust.Although no adverse effects have been reported in the literature among workers exposed to calcium carbonate, high concentrations of the dust would be expected to act as a physical irritant to the eyes and skin.1 Fourteen British workers exposed to heavy calcium carbonate concentrations for 12–35 years showed no trace abnormalities due to dust or any clinical sign of pneumoconiosis or chronic bronchitis on X ray.2 Long exposure to high dust concentrations of pure calcium carbonate (quartz content less than 1.1%) did not result in lung fibrosis.
농업용
Limestone or dolomite, used in agriculture for liming the soil, is ground to a fineness to ensure that 50% of the particles pass through a 1.70mm Indian Standards (IS) sieve, and 50% is retained on a 150micron IS sieve.Safety Profile
A nuisance dust. An eye and skin irritant. Igmtes on contact with F2. Incompatible with acids, ammonium salts, (Mg + H2). Calcium carbonate is a common air contaminant. See also CALCIUM COMPOUNDS.잠재적 노출
Calcium carbonate is used as a source of lime; neutralizing agent; manufacturing or rubber, plastics, paint and coatings; sealants, paper, dentifrices, ceramics, putty, polishes and cleaners, insecticides, inks and cosmetics; whitewash; Portland cement; antacids; analytical chemistry, and others비 호환성
Calcium carbonate decomposes in high temperature forming carbon dioxide and corrosive materials폐기물 처리
Landfills. It is the responsibility of chemical waste generators to determine if a discarded chemical is classified as a hazardous waste. See 40 CFR Parts 261.3 for United States Environmental Protection Agency guidelines for the classification determination. In addition, in order to ensure complete and accurate classification, waste generators must consult state and local hazardous waste regulations.석회석 준비 용품 및 원자재
원자재
준비 용품
Regulus silicate cement
Artinite
붕산나트륨10수화물
ammonia synthesis Fe catalysts
다이사이안 다이아미드
바륨 카보네이트
탄산칼슘
중크롬산나트륨
(R,S)-니코틴
산화칼슘(생석회)
삼산화안티모니
염화칼슘
산화아연
calcium magnesium phosphate(CMP)
실리콘 분
이산화탄소
탄산, 리튬 염
솝스톤
탄화칼슘
AMMONIA SYNTHESIS CATALYST
염화칼슘 2수화물
수산화리튬1수화물
자료없음
칼슘 아질산염
Cement dust
석고
중탄산나트륨(중조)
수산화나트륨
유리솜
아세트산 칼슘
1,2,4-트라이클로로벤젠
차아염소산 칼슘
칼슘 염화물, 헥사수화물
수산화알루미늄분말
칼슘 인산염, 디베이직, 이염화물
수산화칼슘
산화알루미늄
탄산나트륨(경회)
SODA ASH LIGHT 99.2% MIN.
석회석 공급 업체
글로벌( 123)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21691 | 55 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Shanghai Longyu Biotechnology Co., Ltd. | +8615821988213 |
info@longyupharma.com | China | 2531 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 |
sales@chemdad.com | China | 39916 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 |
sales@tnjchem.com | China | 34572 | 58 |
| Hebei Qige Biological Technology Co. Ltd | +86 +8618733132031 |
CHINA | 1313 | 58 | |
| Henan Alfa Chemical Co., Ltd | +8618339805032 |
alfa4@alfachem.cn | China | 12755 | 58 |
| PT CHEM GROUP LIMITED | +86-85511178 +86-85511178 |
peter68@ptchemgroup.com | China | 35453 | 58 |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 |
info@gihichemicals.com | China | 49999 | 58 |
| SUZHOU SENFEIDA CHEMICAL CO.,LTD | +86-0512-83500002 +8615195660023 |
sales@senfeida.com | China | 5100 | 58 |
석회석 관련 검색:
돌로마이트 탄산칼슘 팔라듐 석회석
carbonic acid , barium calcium strontium salt
Lindlarcatalyst
CHALK FRENCH,FRENCH CHALK
Calcite (Ca(CO3))
CALCIUM CARBONATE-13C
kill roach chalk
vanishing chalk
whiting-starch interior wall paint
ARAGONITE, NATURALLY OCCURRING MINERAL, GRAINS, APPROXIMATELY 0.06-0.19IN
IRIDIUM CALCIUM CARBONATE
LIVER CHALK AGAR
PREPARED NATURAL CHALK
MERCURY WITH CHALK
CHALK (CACO2)