아세톤
|
|
아세톤 속성
- 녹는점
- -94 °C(lit.)
- 끓는 점
- 56 °C760 mm Hg(lit.)
- 밀도
- 0.791 g/mL at 25 °C(lit.)
- 증기 밀도
- 2 (vs air)
- 증기압
- 184 mm Hg ( 20 °C)
- FEMA
- 3326 | ACETONE
- 굴절률
- n
20/D 1.359(lit.)
- 인화점
- 1 °F
- 저장 조건
- Store at +5°C to +30°C.
- 용해도
- 물 및 에탄올(96%)과 혼합 가능합니다.
- 산도 계수 (pKa)
- 19.3(at 25℃)
- 물리적 상태
- 액체
- 색상
- 무색, 눈에 보이지 않는 증기
- Specific Gravity
- 0.79 (25/25℃)
- 냄새
- 33~700ppm(평균 = 130ppm)에서 특징적인 자극성 냄새가 감지됩니다.
- 수소이온지수(pH)
- 5-6 (395g/l, H2O, 20°C)
- 상대극성
- 0.355
- 폭발한계
- 2.6-12.8%(V)
- ?? ??
- 용제
- Odor Threshold
- 42ppm
- 수용성
- 녹는
- Merck
- 14,66
- JECFA Number
- 139
- BRN
- 63580
- Henry's Law Constant
- 2.27 at 14.9 °C, 3.03 at 25 °C, 7.69 at 35.1 °C, 11.76 at 44.9 °C (Betterton, 1991)
- 노출 한도
- TLV-TWA 1780 mg/m3 (750 ppm), STEL 2375 mg/m3 (ACGIH); 10 h–TWA 590 mg/m3 (250 ppm); IDLH 20,000 ppm (NIOSH).
- Dielectric constant
- 1.0(0℃)
- LogP
- -0.160
- CAS 데이터베이스
- 67-64-1(CAS DataBase Reference)
- NIST
- Acetone(67-64-1)
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | F,Xi,T | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 11-36-66-67-39/23/24/25-23/24/25 | ||
| 안전지침서 | 9-16-26-45-36/37-7 | ||
| 유엔번호(UN No.) | UN 1090 3/PG 2 | ||
| WGK 독일 | 3 | ||
| RTECS 번호 | AL3150000 | ||
| F 고인화성물질 | 3-10 | ||
| 자연 발화 온도 | 465 °C | ||
| TSCA | Yes | ||
| 위험 등급 | 3 | ||
| 포장분류 | II | ||
| HS 번호 | 29141100 | ||
| 유해 물질 데이터 | 67-64-1(Hazardous Substances Data) | ||
| 독성 | LD50 in rats: 10.7 ml/kg orally (Smyth) | ||
| IDLA | 2,500 ppm | ||
| 기존화학 물질 | KE-29367 |
아세톤 C화학적 특성, 용도, 생산
용도
아세톤은 물질을 녹여 용액으로 만드는 매우 중요한 용매(Solvent)중 하나로 주로 아세톤은 플라스틱과 합성 섬유에 효과적인 용매로 사용되며, 2010년 세계 석유화학 보고서에 따르면 세계에서 제작되는 아세톤의 1/3이 용매로 사용되었습니다.또한 유기 합성의 원료로도 사용되어 용매 또는 시너 등으로 사용되는 다이아세톤 알코올(Diacetone Alcohol)과 같은 화합물의 제작에도 사용되며, 실험실에서는 비커와 같은 유리 제품의 최종 세척전 잔류물과 고형물을 제거하기 위한 세정제로 많이 사용됩니다.
물, 알코올(Alcohol), 에테르(Ether) 등 대부분의 용매와 잘 섞이며, 실생활에서는 이와 같은 성질을 이용하여 페인트나 매니큐어와 같이 물로 세척되지 않는 물질을 세척하는데 사용됩니다.
아세톤은 인화성이 매우 강하기 때문에 불이 잘 붙으며, 폭발의 위험까지 있어 취급시 화기에 멀리 하는 것을 권장합니다.
또한 장기적인 피부 접촉이 있을 경우 심한 염증을 일으킬 수 있는 독성물질에 속합니다.
순도시험
(1) 용해도 : 이 품목 38mL(약 30g에 대응하는 양)를 끓여 식힌 물과 동량의 비로 섞을 때, 그 액은 최소한 30분간 맑아야 한다.
(2) 산도(초산으로서) : 이 품목 38mL를 끓여 식힌 물과 동량의 비로 섞고 0.1N 수산화나트륨용액으로 적정할 때, 그 소비량은 0.1mL 이하이어야 한다(지시약 : 페놀프탈레인시액 0.1mL).
(3) 알칼리도(암모니아로서) : 물 25mL에 메틸레드시액 1방울 가하고 적색이 나타날 때까지 0.1N 황산을 가한 다음 이 품목 23mL(약 18g에 대응하는 양)를 넣고 0.1N 황산으로 적정할 때, 그 소비량은 0.1mL 이하이어야 한다.
(4) 알데히드류(포름알데히드로서) : 이 품목 2.5mL(약 2g에 대응하는 양)를 물 7.5mL에 녹인 액을 시험용액으로 하고 물 10mL에 포름알데히드 40μg을 함유하는 표준용액을 조제한다. 시험용액 및 표준용액에 5% 5,5-이메틸-1,3-시클로헥산디온․에탄올혼액 0.15mL를 각각 가한 후 수욕상에서 아세톤이 휘발할 때까지 증발시킨다. 물을 가하여 10mL로 한 다음 얼음욕조에서 격렬하게 저어주면서 급히 식힐 때, 시험용액의 탁도는 표준용액의 탁도 보다 진하여서는 아니 된다(0.002% 이하).
(5) 과망간산염을 환원하는 물질 : 이 품목 10mL를 마개가 있는 실린더에 넣고 0.1N 과망간산칼륨용액 0.05mL를 가한 다음 15분간 방치할 때, 엷은 적색이 완전히 사라져서는 아니 된다.
(6) 납 : 이 품목 5.0g을 취하여 원자흡광광도법 또는 유도결합플라즈마발광광도법에 따라 시험할 때, 그 양은 1.0ppm 이하이어야 한다.
(7) 메탄올 : 이 품목 10mL에 물을 가하여 100mL로 한 시험용액 및 메탄올표준용액(이 액 1mL는 메탄올 40μg 함유) 각 1mL에 10% 인산 0.2mL 및 과망간산칼륨용액(1→20) 0.25mL를 가한 다음 15분간 방치하고, 아황산수소나트륨용액(1→10) 0.3mL를 가한 후 무색이 될 때까지 잘 흔들어 준다. 얼음에 식힌 80% 황산 5mL를 차게 유지하면서 천천히 가한다. 크로모트로프산용액(1→100) 0.1mL를 가한 다음 수욕상에서 20분간 침지할 때, 시험용액에서 나타나는 보라색이 표준액의 색보다 진하여서는 아니 된다(0.05% 이하).
(8) 페놀류 : 이 품목 3mL를 60℃에서 증발건고하고 이 잔류물에 아질산나트륨의 황산용액(0.1→5) 3방울을 가한 다음 3분간 방치한다. 이 액에 2N 수산화나트륨용액 3mL를 조심스럽게 가할 때, 착색되어서는 아니 된다.
(9) 증류시험 : 이 품목을 비점 및 유분측정법에 따라 유분을 측정할 때, 55.1~57.1℃에서 95%(v/v) 이상을 유출하여야 한다.
(10) 증발잔류물 : 이 품목 125mL(약 100g에 해당하는 양)를 증발건고시킨 다음 105℃에서 30분간 건조할 때, 그 양은 10ppm 이하이어야 한다.
(11) 굴절률 : 이 품목의 굴절률 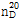 은 1.358~1.360이어야 한다.
은 1.358~1.360이어야 한다.
(12) 비중 : 이 품목의 비중은 0.790~0.793이어야 한다.
확인시험
이 품목 0.1mL를 물 10mL와 섞고 수산화나트륨시액 5mL를 가하여 가온하고 요오드시액 5mL를 가하면 요오드포름의 황색 침전이 생긴다.
정량법
이 품목의 함량은 비중측정법으로 시험한다. 비중으로서 0.7930 이하이어야 한다.
개요
Acetone is a flammable, colorless liquid with a pleasant odor. It is used widely as an organic solvent and in the chemical industry. It is the simplest ketone, which also goes by the name dimethyl ketone (DMK). Acetone was originally referred to as pyroacetic spirit because it was obtained from the destructive distillation of acetates and acetic acid.화학적 성질
Acetone, CH3COCH3, also known as 2-propanone and dimethylketone, is a colorless, volatile,flammable liquid that boils at 56°C (133 OF). It is misciblewith water and is oftenused as a solventin the manufacture of lacquers and paints.물리적 성질
Clear, colorless, liquid with a sweet, fragrant odor. Sweetish taste. Odor threshold concentrations ranged from 42 ppmv (Nagata and Takeuchi, 1990) to 100 ppmv (Leonardos et al., 1969). Experimentally determined detection and recognition odor threshold concentrations were 48 mg/m3 (20 ppmv) and 78 mg/m3 (33 ppmv), respectively (Hellman and Small, 1974).출처
Reported found in apple, pear, grape, pineapple, strawberry, raspberry, tomato, black currant, citrus, onion and potato; also reported found in cocoa leaves, in Mexican goosefoot and in the oils of coriander and lavender. In trace amounts it has been reportedly identified in the oil of bitter orange, in distilled wine and in coffee aroma역사
The traditional method of producing acetone in the 19th century and the beginning of the 20th century was to distill acetates, particularly calcium acetate, Ca(C2H3O2)2.Weizmann discovered a process to produce butyl alcohol and acetone from the bacterium Clostridium acetobutylicum in 1914. With England’s urgent demand for acetone, Winston Churchill (1874–1965) enlisted Weizmann to develop the Weizmann process for acetone production on an industrial scale. Fermentation and distillation techniques for acetone production were replaced starting in the 1950s with the cumene oxidation process . In this process, cumene is oxidized to cumene hydroperoxide, which is then decomposed using acid to acetone and phenol. This is the primary method used to produce phenol, and acetone is produced as a co-product in the process, with a yield of about 0.6:1 of acetone to phenol.
용도
Acetone′s luminesence intensity is dependent upon the solution components 1. The absorption of UV light by acetone, results in its photolysis and the production of radials 1.정의
ChEBI: A methyl ketone that consists of propane bearing an oxo group at C2.생산 방법
Acetone is obtained by fermentation as a by-product of n-butyl alcohol manufacture, or by chemical synthesis from isopropyl alcohol; from cumene as a by-product in phenol manufacture; or from propane as a by-product of oxidation-cracking.일반 설명
A clear colorless liquid with a sweetish odor. Flash point 0°F. Less dense than water. Vapors are heavier than air. Used as a solvent in paint and nail polish removers.공기와 물의 반응
Highly flammable. Water soluble.반응 프로필
Acetone was reported that a mixture of Acetone and chloroform, in a residue bottle, exploded. Since addition of Acetone to chloroform in the presence of base will result in a highly exothermic reaction, Acetone is thought that a base was in the bottle [MCA Case History 1661. 1970]. Also, Nitrosyl chloride, sealed in a tube with a residue of Acetone in the presence of platinum catalyst, gave an explosive reaction [Chem. Eng. News 35(43):60. 1967]. The reaction of nitrosyl perchlorate and Acetone ignites and explodes. Explosions occur with mixtures of nitrosyl perchlorate and primary amine [Ann. Chem. 42:2031. 1909]. Reacts violently with nitric acid. Also causes exothermic reaction when in contact with aldehydes.건강위험
The acute toxicity of acetone is low. Acetone is primarily a central nervous system depressant at high concentrations (greater than 12,000 ppm). Unacclimated volunteers exposed to 500 ppm acetone experienced eye and nasal irritation, but it has been reported that 1000 ppm for an 8-hour day produced no effects other than slight transient irritation to eyes, nose, and throat. Therefore there are good warning properties for those unaccustomed to working with acetone; however, frequent use of acetone seems to cause accommodation to its slight irritating properties. Acetone is practically nontoxic by ingestion. A case of a man swallowing 200 mL of acetone resulted in his becoming stuporous after 1 hour and then comatose; he regained consciousness 12 hour later. Acetone is slightly irritating to the skin, and prolonged contact may cause dermatitis. Liquid acetone produces moderate transient eye irritation. Acetone has not been found to be carcinogenic in animal tests or to have effects on reproduction or fertility.화재위험
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.인화성 및 폭발성
Acetone is extremely flammable (NFPA rating = 3), and its vapor can travel a considerable distance to an ignition source and "flash back." Acetone vapor forms explosive mixtures with air at concentrations of 2 to 13% (by volume). Carbon dioxide or dry chemical extinguishers should be used for acetone fires.Pharmaceutical Applications
Acetone is used as a solvent or cosolvent in topical preparations, and as an aid in wet granulation.It has also been used when formulating tablets with water-sensitive active ingredients, or to solvate poorly water-soluble binders in a wet granulation process. Acetone has also been used in the formulation of microspheres to enhance drug release.Owing to its low boiling point, acetone has been used to extract thermolabile substances from crude drugs.공업 용도
Acetone is valuable solvent component in acrylic/nitrocellulose automotive lacquers. Acetone is the solvent of choice in film coatings operations which use vinylidene chloride-acrylonitrile copolymer formulations.Other ketones that may be used in these film coating operations include methyl isobutyl ketone, ethyl n-amyl ketone, and diisobutyl ketone.Acetone, blends of MIBK and MEK, methyl namyl ketone, ethyl n-amyl ketone, and diisobutyl ketone are all useful solvents for vinyl resin copolymers. The presence of one of the slower evaporating ketones in the solvent blend prevents quick drying, improves flow, and gives blush resistance to the coating. Acetone is also used as a resin thinner in polyester resins and as a clean up solvent for the resin reactor kettle.In solvents industry, Acetone is a component of solvent blends in urethane, nitrile rubber, and neoprene industrial adhesives.Acetone is the primary solvent in resin-type adhesives and pressure sensitive chlorinated rubber adhesives. Acetone also can be used to extract fats, oils, waxes, and resins from natural products, to dewax lubricating oils, and to extract certain essential oils.Acetone is also an important chemical intermediate in the preparation of several oxygenated solvents including the ketones, diacetone alcohol, mesityl oxide, methyl isobutyl ketone, and isophorone.Safety
Acetone is considered moderately toxic, and is a skin irritant and severe eye irritant. Skin irritation has been reported due to its defatting action, and prolonged inhalation may result in headaches. Inhalation of acetone can produce systemic effects such as conjunctival irritation, respiratory system effects, nausea, and vomiting.LD50 (mouse, oral): 3.0 g/kg
LD50 (mouse, IP): 1.297 g/kg
LD50 (rabbit, oral): 5.340 g/kg
LD50 (rabbit, skin): 0.2 g/kg
LD50 (rat, IV): 5.5 g/kg
LD50 (rat, oral): 5.8 g/kg
잠재적 노출
It is used as a solvent in nail polish remover and many other chemicals. Used in the production of lubricating oils and as an intermediate in the manufacture of chloroform and of various pharmaceuticals and pesticides.Carcinogenicity
Acetone may be weakly genotoxic, but the majority of assays were negative.7 It was not tumorigenic in skin painting studies in mice.The 2003 ACGIH threshold limit valuetime- weighted average (TLV-TWA) for acetone is 750ppm (1780mg/m3) with a short-term excursion level of 1000ppm (2380mg/m3).
환경귀착
Biological. Following a lag time of 20 to 25 h, acetone degraded in activated sludge (30 mg/L) at a rate constant ranging from 0.016 to 0.020/h (Urano and Kato, 1986). Soil bacteria can mineralize acetone to carbon dioxide (Taylor et al., 1980). Bridié et al. (1979) reported BOD and COD values of 1.85 and 1.92 g/g using filtered effluent from a biological sanitary waste treatment plant. These values were determined using a standard dilution method at 20 °C for a period of 5 d. Similarly, Heukelekian and Rand (1955) reported a 5-d BOD value of 0.85 g/g which is 38.5% of the ThOD value of 2.52 g/g. Waggy et al. (1994) reported 5, 15, and 28-d BOD values of 14, 74, and 74%, respectively. Using the BOD technique to measure biodegradation, the mean 5-d BOD value (mM BOD/mM acetone) and ThOD were 1.52 and 38.0%, respectively (Vaishnav et al., 1987).Photolytic. Photolysis of acetone in air yields carbon monoxide and free radicals, but in isopropanol, pinacol is formed (Calvert and Pitts, 1966). Photolysis of acetone vapor with nitrogen dioxide via a mercury lamp gave peroxyacetyl nitrate as the major product with smaller quantities of methyl nitrate (Warneck and Zerbach, 1992).
Chemical/Physical. Hypochlorite ions, formed by the chlorination of water for disinfection purposes, may react with acetone to form chloroform. This reaction is expected to be significant within the pH range of 6 to 7 (Stevens et al., 1976).








