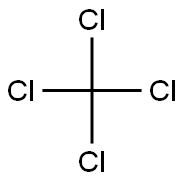
사염화탄소
|
|
사염화탄소 속성
- 녹는점
- -23 °C
- 끓는 점
- 76-77 °C(lit.)
- 밀도
- 1.594 g/mL at 25 °C(lit.)
- 증기 밀도
- 5.32 (vs air)
- 증기압
- 4.05 psi ( 20 °C)
- 굴절률
- n
20/D 1.460(lit.)
- 인화점
- −2 °F
- 저장 조건
- 2-8°C
- 용해도
- Miscible with ethanol, benzene, chloroform, ether, carbon disulfide (U.S. EPA, 1985), petroleum ether, solvent naphtha, and volatile oils (Yoshida et al., 1983a).
- 물리적 상태
- 액체
- 색상
- 무색의
- 냄새
- 140~584ppm(평균 = 252ppm)에서 감지 가능한 미묘하고 달콤하며 자극적인 냄새
- 상대극성
- 0.052
- Odor Threshold
- 4.6ppm
- 수용성
- 0.8g/L(20℃)
- 최대 파장(λmax)
- λ: 265 nm Amax: 1.0
λ: 270 nm Amax: 0.30
λ: 280 nm Amax: 0.07
λ: 290 nm Amax: 0.02
λ: 300-400 nm Amax: 0.01
- Merck
- 13,1826
- BRN
- 1098295
- Henry's Law Constant
- 2.15 at 30 °C (headspace-GC, Sanz et al., 1997)
- 노출 한도
- NIOSH REL: STEL 1 hour 2 ppm, IDLH 200 ppm; OSHA PEL: TWA 10 ppm, C 25 ppm, 5-minute/4-hour peak 200 ppm; ACGIH TLV: TWA 5 ppm.
- Dielectric constant
- 2.2(20℃)
- InChIKey
- VZGDMQKNWNREIO-UHFFFAOYSA-N
- LogP
- 2.830
- CAS 데이터베이스
- 56-23-5(CAS DataBase Reference)
- IARC
- 2B (Vol. 20, Sup 7, 71) 1999
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | T,N,F | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 23/24/25-40-48/23-52/53-59-39/23/24/25-11-43 | ||
| 안전지침서 | 23-36/37-45-59-61-16-7 | ||
| 유엔번호(UN No.) | UN 1846 6.1/PG 2 | ||
| WGK 독일 | 3 | ||
| RTECS 번호 | FG4900000 | ||
| F 고인화성물질 | 8-9 | ||
| 위험 등급 | 6.1(a) | ||
| 포장분류 | II | ||
| HS 번호 | 29031400 | ||
| 유해 물질 데이터 | 56-23-5(Hazardous Substances Data) | ||
| 독성 | LC50 for mice: 9528 ppm (Svirbely); LD50 in rats, mice, dogs (g/kg): 2.92, 12.1-14.4, 2.3 orally; LD50 in mice (g/kg): 4.1 i.p., 30.4 s.c. (IARC, 1979) | ||
| IDLA | 200 ppm | ||
| 기존화학 물질 | KE-04756 | ||
| 유해화학물질 필터링 | 97-1-126;06-5-3 | ||
| 중점관리물질 필터링 | 별표1-5 | ||
| 함량 및 규제정보 | 물질구분: 유독물질; 혼합물(제품)함량정보: 사염화 탄소 및 이를 1% 이상 함유한 혼합물 |
사염화탄소 C화학적 특성, 용도, 생산
개요
Carbon tetrachloride is a manufactured chemical and does not occur naturally in the environment. It is produced by chlorination of a variety of low molecular weight hydrocarbons such as carbon disulfide, methane, ethane, propane, or ethylene dichloride and also by thermal chlorination of methyl chloride. Carbon tetrachloride is a precursor for chlorofluorocarbon (CFC) gases that have been used as aerosol propellant. A decrease in this use is occurring due to the agreement reached in the Montreal Protocol for the reduction of environmental concentrations of ozone-depleting chemicals, including carbon tetrachloride.화학적 성질
Carbon tetrachloride, CC14, also known as tetrachloromethane, perchloro methane, and benzinoform, is a colorless liquid with a boiling point of 77 °C (170 OF). It is used as a solvent for lacquers, resin, and rubbers,and as a dry cleaning agent.물리적 성질
Carbon tetrachloride is a volatile colourless clear heavy liquid with a characteristic sweet non-irritant odour. The odour threshold in water is 0.52 mg/litre and in air is > 10 ppm. Carbon tetrachloride is miscible with most aliphatic solvents and it is a solvent for benzyl resins, bitumen, chlorinated rubber, rubber-based gums, oils and fats.The solubility in water is low. Carbon tetrachloride is non-flammable and is stable in the presence of air and light. Decomposition may produce phosgene, carbon dioxide and hydrochloric acid.역사
In the 1890s, commercial manufacturing processes were being investigated by the United Alkali Co. in England. At the same time it was also produced in Germany, exported to the United States, and retailed as a spotting agent under the trade name Carbona. Large-scale production of carbon tetrachloride in the United States commenced in the early 1900s. By 1914, annual production fell just short of 4500 metric tons and was used primarily for dry cleaning and for charging fire extinguishers. During World War I, U.S. production of carbon tetrachloride expanded greatly; its use was extended to grain fumigation and the rubber industry. In 1934 it was supplanted as the predominant dry-cleaning agent in the United States by perchloroethylene, which is much less toxic and more stable. During the years immediately preceding World War II, trichloroethylene began to displace carbon tetrachloride from its then extensive market in the United States as a metal degreasing solvent. Carbon tetrachloride is more difficult to recover from degreasing operations, more readily hydrolyzed, and more toxic than trichloroethylene C2HCl3. The demands of World War II stimulated production and marked the beginning of its use as the starting material for chlorofluoromethanes, by far the most important application for carbon tetrachloride.용도
Carbon tetrachloride is used as a solvent, infire extinguishers, in dry cleaning, and in themanufacture of fluorocarbon propellents.As solvent for oils, fats, lacquers, varnishes, rubber waxes, resins; starting material in manufacture of organic Compounds. Pharmaceutic aid (solvent).정의
ChEBI: A chlorocarbon that is methane in which all the hydrogens have been replaced by chloro groups.생산 방법
Carbon tetrachloride is made by the reaction of carbon disulfide and chlorine in the presence of a catalyst, such as iron or antimony pentachloride:CS2 + 3Cl2 → CCl4 + S2Cl2
Sulfur chloride is removed by treatment with caustic soda solution. The product is purified by distillation.
Alternatively, CCl4 may be prepared by heating a mixture of chlorine and methane at 250 to 400°C.
CH4 + 4Cl2 → CCl4 + 4HCl
일반 설명
Carbon tetrachloride appears as a clear colorless liquid with a characteristic odor. Denser than water (13.2 lb / gal) and insoluble in water. Noncombustible. May cause illness by inhalation, skin absorption and/or ingestion. Used as a solvent, in the manufacture of other chemicals, as an agricultural fumigant, and for many other uses.공기와 물의 반응
Insoluble in water.반응 프로필
Carbon tetrachloride is a commonly used liquid in fire extinguishers to combat small fires. Carbon tetrachloride has no flash point, Carbon tetrachloride is not flammable. However, when heated to decomposition, Carbon tetrachloride will emit fumes of extremely toxic phosgene and of hydrogen chloride. Forms explosive mixtures with chlorine trifluoride, calcium hypochlorite, decaborane, dinitrogen tetraoxide, fluorine. Forms impact-sensitive explosive mixtures with particles of many metals: lithium, sodium, potassium, beryllium, zinc, aluminum, barium. Vigorous exothermic reaction with allyl alcohol, boron trifluoride, diborane, disilane, aluminum chloride, dibenzoyl peroxide, potassium tert-butoxide, liquid oxygen, zirconium. [Bretherick, 5th ed., 1995, p. 666]. Potentially dangerous reaction with dimethylformamide or dimethylacetamide in presence of iron [Cardillo, P. et al., Ann. Chim. (Rome), 1984, 74, p. 129].위험도
Carbon tetrachloride is a poison and also a carcinogen. The acute toxicity of this compound in humans is of low order. However, the ingestion of the liquid can be fatal, death resulting from acute liver or kidney necrosis. (Patnaik, P. 1999. A Comprehensive Guide to the Hazardous Properties of Chemical Substances, 2nd ed. New York: John Wiley & Sons.) The acute poisoning effects are headache, dizziness, fatigue, stupor, nausea, vomiting, diarrhea, and liver damage. Chronic exposure can damage both liver and kidney. Carbon tetrachloride also is a suspected human carcinogen. It causes liver and thyroid cancers in experimental animals.인화성 및 폭발성
Carbon tetrachloride is noncombustible. Exposure to fire or high temperatures may lead to formation of phosgene, a highly toxic gas.공업 용도
Carbon tetrachloride is a clear, heavy liquid with a strong, aromatic odor. Its formula is CC14. It is produced in large quantities for use in the manufacturing of refrigerants and propellants for aerosol cans. It is also used as a feedstock in the synthesis of chlorofluorocarbons and other chemicals, in petroleum refining, pharmaceutical manufacturing, and general solvent use. Until the mid- 1960s, it was also widely used as a cleaning fluid, both in industry, where it served as a degreasing agent, and in the home, where it was used as a spot remover and in fire extinguishers.Carbon tetrachloride is a highly volatile liquid with a strong etherial odor similar to chloroform. It mixes sparingly with water and is not flammable. When heated to decomposition, it emits highly toxic fumes of phosgene and hydrogen chloride. There is strong evidence that the toxicity of carbon tetrachloride is dramatically increased by its interaction with alcohols, ketones, and a range of other chemicals.
Carbon tetrachloride is known to deplete the ozone layer, where it is responsible for 17% of the ozone-destroying chlorine now in the stratosphere due to human activities. Carbon tetrachloride has a half-life of between 30 and 100 years.Its DOT Label is Poison, and its UN number is 1846.
Safety Profile
Also forms explosive mixtures with chlorine trifluoride, calcium hypochlorite (heatsensitive), calcium dtsllicide (frictionand pressuresensitive), triethyldialuminum trichloride (heatsensitive), decaborane(l4) (impact-sensitive), dinitrogen tetraoxide. Violent or explosive reaction on contact with fluorine. Forms explosive mixtures with ethylene between 25' and 105' and between 30 and 80 bar. Potentially explosive reaction on contact with boranes. 9:l mixtures of methanol and cCl4 react exothermically with aluminum, magnesium, or zinc. Potentially dangerous reaction with dimethyl formamide, 1,2,3,4,5,6 hexachlorocyclohexane, or dtmethylacetamide when iron is present as a catalyst. CCh has caused explosions when used as a fire extingusher on wax and uranium fires. Incompatible with aluminum trichloride, dtbenzoyl peroxide, potassiumtert-butoxide. Vigorous exothermic reaction with allyl alcohol, Al(C2H5)3, (benzoyl peroxide + C2H4), BrF3, diborane, dsilane, liquid O2, Pu, (AgClO4 + HCl), potassiumtert-butoxide, tetraethylenepentamine, tetrasilane, trisilane, Zr. When heated to decomposition it emits toxic fumes of Cl and phosgene. It has been banned from household use by the FDA.잠재적 노출
Carbon tetrachloride, and organochlorine, is used as a solvent for oils, fats, lacquers, varnishes, rubber, waxes, and resins. Fluorocarbons are chemically synthesized from it. It is also used as an azeotropic drying agent for spark plugs; a dry-cleaning agent; a fire extinguishing agent; a fumigant, and an anthelmintic agent. The use of this solvent is widespread, and substitution of less toxic solvents when technically possible is recommended.Carcinogenicity
Carbon tetrachloride is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals.저장
Carbon tetrachloride should be handled in the laboratory using the "basic prudent practices".운송 방법
UN1846 Carbon tetrachloride, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.비 호환성
Oxidative decomposition on contact with hot surfaces, flames, or welding arcs. Carbon tetrachloride decomposes forming toxic phosgene fumes and hydrogen chloride. Decomposes violently (producing heat) on contact with chemically active metals, such as aluminum, barium, magnesium, potassium, sodium, fluorine gas, allyl alcohol, and other substances, causing fire and explosion hazard. Attacks copper, lead, and zinc. Attacks some coatings, plastics, and rubber. Becomes corrosive when in contact with water; corrosive to metals in the presence of moisture.폐기물 처리
Incineration, preferably after mixing with another combustible fuel; care must be exercised to assure complete combustion to prevent the formation of phosgene; an acid scrubber is necessary to remove the halo acids produced. Recover and purify by distillation where possible.사염화탄소 준비 용품 및 원자재
원자재
준비 용품
3,4-DIBROMO-4-PHENYL-2-BUTANONE
2-Nitrobenzenesulfenyl chloride
2,5-DICHLOROBENZOYL CHLORIDE
DIMETHYL 4-CHLOROPYRIDINE-2,6-DICARBOXYLATE
2,4-Dichlorobenzotrifluoride
2,6-Dibromopyridin-3-amine
1-(4-BROMO-2-THIENYL)ETHAN-1-ONE
1-브로모-2,4-디플로로벤젠
N-Chloromethyl-N-phenylaminoformyl chloride
Ethyl 2-amino-4-phenyl-5-thiazolecarboxylate
2-(Chloromethyl)pyridine
고무, 클로리네이티드
METHYL 4-(BROMOMETHYL)-3-METHOXYBENZOATE
테트라아이오딘화 탄소
다이아세톤 아크릴라마이드
2,3-디클로로-5,6-디시아노-1,4-벤조퀴논
2,5-디메틸티오펜
2-(Bromomethyl)pyridine hydrobromide
6-BROMOMETHYL-2-PYRIDINECARBOXYLIC ACID
9,10-Dibromoanthracene
3,5-DI-T-BUTYL-4-METHOXYBENZALDEHYDE
Atropine sulfate monohydrate
2-(AMINOCARBONYL)NICOTINIC ACID
3-Bromonitrobenzene
2,3-디브로모프로파놀
2-(2-BROMOACETYL)THIOPHENE
4-BROMO-2.6-DICHLOROPHENOL
Bromotrichloromethane
1,2,3,4-Tetrachloro-5,6-Dimethylbenzylene
1-(BROMOMETHYL)-4-(METHYLSULFONYL)BENZENE
Dimethylthiocarbamoyl chloride
5-BROMO-2-(METHYLTHIO)PYRIMIDINE
살리실아닐라이드
Diphenyldichloromethane
5-IODOCYTOSINE
IMIDAZO[1,2-A]PYRIDINE-2-CARBOXYLIC ACID ETHYL ESTER
벤조산노르말부틸에스테르
2-Nitrobenzyl bromide
5-BROMO-2-METHANESULFONYL-PYRIMIDINE
크로톤산메틸에스테르