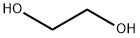모노에틸렌글리콜
|
|
모노에틸렌글리콜 속성
- 녹는점
- -13 °C (lit.)
- 끓는 점
- 195-198 °C
- 밀도
- 1.113 g/mL at 25 °C (lit.)
- 증기 밀도
- 2.1 (vs air)
- 증기압
- 0.08 mm Hg ( 20 °C)
- 굴절률
- n
20/D 1.431(lit.)
- 인화점
- 230 °F
- 저장 조건
- 2-8°C
- 용해도
- 물: 섞일 수 있음
- 물리적 상태
- 점성 액체
- 산도 계수 (pKa)
- 14.22(at 25℃)
- 색상
- 파란색
- 냄새
- 냄새 없는
- 상대극성
- 0.79
- 수소이온지수(pH)
- 6-7.5 (100g/l, H2O, 20℃)
- 폭발한계
- 3.2%(V)
- 수용성
- 혼용 가능
- 어는점
- -11.5℃
- 감도
- Hygroscopic
- 최대 파장(λmax)
- λ: 260 nm Amax: ≤0.03
λ: 280 nm Amax: ≤0.01
- Merck
- 14,3798
- BRN
- 505945
- 노출 한도
- Ceiling limit in air for vapor and mist 50 ppm (~125 mg/m3) (ACGIH); TWA 10 mg/m3 (particulates) (MSHA).
- Dielectric constant
- 37.0(20℃)
- LogP
- -1.36 at 25℃
- CAS 데이터베이스
- 107-21-1(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xn | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 22-36-41 | ||
| 안전지침서 | 26-39-36/37/39 | ||
| WGK 독일 | 3 | ||
| RTECS 번호 | KW2975000 | ||
| 자연 발화 온도 | 752 °F | ||
| TSCA | Yes | ||
| HS 번호 | 29053100 | ||
| 유해 물질 데이터 | 107-21-1(Hazardous Substances Data) | ||
| 독성 | LD50 in rats, guinea pigs (g/kg): 8.54, 6.61 orally (Smyth); in mice (ml/kg): 13.79 orally (Bornmann) | ||
| 기존화학 물질 | KE-13169 |
모노에틸렌글리콜 C화학적 특성, 용도, 생산
개요
Ethylene glycol was first synthesized in 1859; however, it did not become a public health concern until after World War II. In fact, the first published series of deaths from ethylene glycol consumption involved 18 soldiers who drank antifreeze as a substitute for ethanol. Despite the early recognition that patients who drank ethanol in addition to ethylene glycol had prolonged survival when compared to those drinking ethylene glycol alone, antidotal treatment of ethylene glycol toxicity with ethanol was not evaluated until the 1960s. Today, ethylene glycol poisoning continues to be a public health problem, particularly in the southeastern United States. In 2009, US poison control centers received 5282 calls about possible ethylene glycol exposures, and the toxicology community believes these exposures are underreported.화학적 성질
Ethylene glycol,CH20HCH20H, also known as glycol,ethylene alcohol, glycol alcohol, and dihydric alcohol, is a colorless liquid. It is soluble in water and in alcohol. Ethyleneglycol has a low freezing point,-25°C (-13 OF), and is widely used as an antifreeze in automobiles and in hydraulic fluids. It is used as a solvent for nitrocellulose and in the manufacture of acrylonitrile, dynamites, and resins.용도
Ethylene glycol is used as an antifreeze inheating and cooling systems (e.g., automobileradiators and coolant for airplane motors).It is also used in the hydraulic brake fluids;as a solvent for paints, plastics, and inks; as a softening agent for cellophane; and in themanufacture of plasticizers, elastomers, alkydresins, and synthetic fibers and waxes.생산 방법
Historically, ethylene glycol has been manufactured by hydrolyzing ethylene oxide. Presently, it is also produced commercially by oxidizing ethylene in the presence of acetic acid to form ethylene diacetate, which is hydrolyzed to the glycol, and acetic acid is recycled in the process .정의
ChEBI: A 1,2-glycol compound produced via reaction of ethylene oxide with water.제조 방법
Ethylene glycol is prepared by the hydration of ethylene oxide:
This reaction is carried out in a manner comparable to that described for the preparation of propylene glycol from propylene oxide . Ethylene glycol is a colourless liquid, b.p. 197??C.
화학 반응
Glycol reacts (1) with sodium to form sodium glycol, CH2OH · CH2ONa, and disodium glycol, CH2ONa·CH2ONa; (2) with phosphorus pentachloride to form ethylene dichloride, CH2Cl·CH2Cl (3) with carboxy acids to form mono- and disubstituted esters, e.g., glycol monoacetate, CH2OH·CH2OOCCH3, glycol diacetate, CH3COOCH2 · CH2OOCCH3; (4) with nitric acid (with sulfuric acid), to form glycol mononitrate, CH2OH·CH2ONO2, glycol dinitrate, CH2ONO2 · CH2ONO2; (5) with hydrogen chloride, heated, to form glycol chlorohydrin (ethylene chlorohydrin, CH2OH·CHCl); (6) upon regulated oxidation to form glycollic aldehyde, CH2OH·CHO, glyoxal, CHO · CHO, glycollic acid, CH2OH·COOH, glyoxalic acid, CHO·COOH, oxalic acid, COOH·COOH.일반 설명
Ethylene glycol is a clear, colorless syrupy liquid. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. Since Ethylene glycol is a liquid Ethylene glycol can easily penetrate the soil and contaminate groundwater and nearby streams.반응 프로필
Mixing Ethylene glycol in equal molar portions with any of the following substances in a closed container caused the temperature and pressure to increase: chlorosulfonic acid, oleum, sulfuric acid, [NFPA 1991].위험도
Questionable carcinogen. Toxic by ingestion and inhalation. Lethal dose reported to be 100 cc.건강위험
Inhalation of vapor is not hazardous. Ingestion causes stupor or coma, sometimes leading to fatal kidney injury.화재위험
Ethylene glycol is combustible.Safety Profile
Human poison by ingestion. (Lethal dose for humans reported to be 100 mL.) Moderately toxic to humans by an unspecified route. Moderately toxic experimentally by ingestion, subcutaneous, intravenous, and intramuscular routes. Human systemic effects by ingestion and inhalation: eye lachrymation, general anesthesia, headache, cough, respiratory stimulation, nausea or vomiting, pulmonary, kidney, and liver changes. If ingested it causes initial central nervous system stimulation followed by depression. Later, it causes potentially lethal kidney damage. Very toxic in particulate form upon inhalation. An experimental teratogen. Other experimental reproductive effects. Human mutation data reported. A skin, eye, and mucous membrane irritant. Combustible when exposed to heat or flame; can react vigorously with oxidants. Moderate explosion hazard when exposed to flame. Iptes on contact with chromium trioxide, potassium permanganate, and sodium peroxide. Mixtures with ammonium dichromate, silver chlorate, sodium chlorite, and uranyl nitrate ipte when heated to 100°C. Can react violently with chlorosulfonic acid, oleum, H2SO4, HClO4, and Pass. Aqueous solutions may ignite silvered copper wires that have an applied D.C. voltage. To fight fire, use alcohol foam, water, foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes.잠재적 노출
Ethylene glycol is used in antifreeze (especially as car radiator antifreeze) and in production of polyethylene terephthalate fibers and films; in hydraulic fluids; antifreeze and coolant mixtures for motor vehicles; electrolytic condensers; and heat exchangers. It is also used as a solvent and as a chemical intermediate for ethylene glycol dinitrate, glycol esters; resins, and for pharmaceuticals.환경귀착
Ethylene glycol is considered an inert ingredient in pesticides. It typically enters the environment through waste streams after use of deicing products, where it is highly mobile in soil and contaminates groundwater. Ethylene glycol is considered ‘readily biodegradable.’ It biodegrades relatively quickly; its half-life (t1/2) is 2–12 days in soil.Ethylene glycol is biodegraded in water under both aerobic and anaerobic conditions within a day to a few weeks. In the atmosphere, ethylene glycol photochemically degrades with a t1/2 of approximately 2 days.
Solubility in organics
Miscible with water and alcohol, soluble in lower atifatic alcohols and ketones, Propylene glycol and Glycerin, poorly soluble in Hydrocarbons such as Terpenes as well as in Terpene alcohols, esters, etc.운송 방법
UN3082 Environmentally hazardous substances, liquid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name RequiredPurification Methods
It is very hygroscopic, and also likely to contain higher diols. Dry it with CaO, CaSO4, MgSO4 or NaOH and distil it under vacuum. Dry further by reaction with sodium under nitrogen, reflux for several hours and distil. The distillate is then passed through a column of Linde type 4A molecular sieves and finally distil under nitrogen, from more molecular sieves. Then fractionally distil it. [Beilstein 1 IV 2369.]비 호환성
Reacts with sulfuric acid, oleum, chlorosulfonic acid; strong oxidizing agents; strong bases; chromium trioxide; potassium permanganate; sodium peroxide. Hygroscopic (i.e., absorbs moisture from the air)폐기물 처리
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed. Alternatively, ethylene glycol can be recovered from polyester plant wastes모노에틸렌글리콜 준비 용품 및 원자재
원자재
준비 용품
2,4-DIAMINO-6-MERCAPTOPYRIMIDINE
2-브로모에틸-2-에틸-1,3-디옥소란
tussah silk fabric aftertreatment finishing agent
루미놀
옥살산
2-에틸-5-메틸티오펜
4-포르밀페닐보론산
5-클로로-1,3-다이하이드로-2H-인돌-2-온
Polyester Filament
PolyesterPolyol
3-벤조일프로피오페논
Dye-fixing agent,no formaldehyde
solid alcohol
2-피로리딘온,1-에테닐-
6-아자티민
21-Iodo-16-methylpregna-1,4,9(11)-trien-17-ol-3,20-dione
에틸렌 글리콜 다이글리시딜 에테르
클로로-2-메틸-1-프로펜
2,3-티오펜디카르복스알데히드
4-(Hydroxymethyl)phenylboronic acid
에틸렌브라실레이트
2-(2,4-DINITROPHENOXY)ETHANOL
Antifreeze
16,17-에폭시프레그나-5,9(11)-디엔-3,20-디온고리형비스(1,2-에탄디일아세탈)
Saponified soluble oil
16-Methylpregna-1,4,9(11)-trien-17-ol-3,20-dione
9-Bromo-11,17,21-trihydroxy-16-methylpregna-1,4-diene-3,20-dione-21-acetate
16-Methylpregna-4,9(11)-dien-17-ol-3,20-dione
17-Ethinyl-17-hydroxy-18-methylestra-5(10),9(11)-dien-3-one-3-ethylene ketal
17,21-dihydroxy-16beta-methylpregna-1,4,9(11)-triene-3,20-dione 21-acetate
4,5-DIMETHYLTHIOPHENE-2-카르복실산
5-[(1,3-DIOXO-1,3-DIHYDRO-2H-ISOINDOL-2-YL)METHYL]-2-FURALDEHYDE
2,4-DIMETHYLTHIOPHENE
2,3-Dimethylthiophene
2-페닐-2-이미다졸린
4,5-디메틸티오펜-2-카르복스알데히드
에틸렌 플루오로히드린
덱사메타손 아세트산, 무수물
9,11β-Epoxy-17-hydroxy-16β-methyl-3,20-dioxo-9β-pregna-1,4-diene-21-yl Acetate
C.I. 염료 황색 185
모노에틸렌글리콜 공급 업체
글로벌( 1381)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Shandong Deshang Chemical Co., Ltd. | +86-0531-8875-2665 +8613153039501 |
info@deshangchem.com | China | 646 | 58 |
| Shandong Zhishang New Material Co., Ltd. | +8617653113209 |
sales002@sdzschem.com | China | 3050 | 58 |
| PT CHEM GROUP LIMITED | |
peter68@ptchemgroup.com | China | 35426 | 58 |
| Shanghai Bojing Chemical Co.,Ltd. | +86-86-02137122233 +8613795318958 |
bj1@bj-chem.com | China | 299 | 55 |
| Yujiang Chemical (Shandong) Co.,Ltd. | +86-17736087130 +86-18633844644 |
catherine@yjchem.com.cn | China | 982 | 58 |
| Aladdin Scientific | +1-+1(833)-552-7181 |
sales@aladdinsci.com | United States | 57511 | 58 |
| Shandong Dexiang International Trade Co., Ltd | +8615662695772 |
2476434775@qq.com | China | 998 | 58 |
| SHANGHAI KEAN TECHNOLOGY CO., LTD. | +8613817748580 |
cooperation@kean-chem.com | China | 40068 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 |
sales@hbmojin.com | China | 12446 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 |
sales1@chuanghaibio.com | China | 5872 | 58 |