다이에틸렌 글리콜
|
|
다이에틸렌 글리콜 속성
- 녹는점
- −10 °C(lit.)
- 끓는 점
- 245 °C(lit.)
- 밀도
- 1.118 g/mL at 25 °C(lit.)
- 증기 밀도
- 2.14 (vs air)
- 증기압
- 0.01 mm Hg ( 20 °C)
- 굴절률
- n
20/D 1.447(lit.)
- 인화점
- 143 °C
- 저장 조건
- Keep in dark place,Sealed in dry,Room Temperature
- 용해도
- H2O: 50 mg/mL at 20 °C, 투명, 무색
- 물리적 상태
- 기름진 액체
- 산도 계수 (pKa)
- 14.03±0.10(Predicted)
- 색상
- 무색의
- 상대극성
- 0.713
- 수소이온지수(pH)
- 5.5-7.0 (25℃, 50mg/mL in H2O)
- 냄새
- 거의 무취.
- 폭발한계
- 2-12.3%
- 수용성
- 녹는
- 어는점
- -10.45℃
- 감도
- Hygroscopic
- 최대 파장(λmax)
- λ: 260 nm Amax: ≤0.02
λ: 280 nm Amax: ≤0.01
- Merck
- 14,3119
- BRN
- 969209
- Dielectric constant
- 31.7(20℃)
- 안정성
- 흡습성
- InChIKey
- MTHSVFCYNBDYFN-UHFFFAOYSA-N
- LogP
- -1.98 at 20℃
- CAS 데이터베이스
- 111-46-6(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xn,T,Xi | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 22 | ||
| 안전지침서 | 46 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | ID5950000 | ||
| F 고인화성물질 | 10 | ||
| 자연 발화 온도 | 442 °F | ||
| 위험 참고 사항 | Toxic/Irritant | ||
| TSCA | Yes | ||
| HS 번호 | 29094100 | ||
| 유해 물질 데이터 | 111-46-6(Hazardous Substances Data) | ||
| 독성 | LD50 in rats, guinea pigs (g/kg): 20.76, 13.21 orally (Smyth) | ||
| 기존화학 물질 | KE-27694 |
다이에틸렌 글리콜 C화학적 특성, 용도, 생산
물성
흡습성으로 거의 냄새가 없는 점조한 조금 단맛이 나는 액체. 응고점 -6.5℃, 끓는점 245℃, 133℃/14mm, 119~120℃/7mm. 1.1197, 1.118, 1.4488. 물, 에탄올, 에테르, 아세톤, 에틸렌글리콜에 녹는다. 벤젠, 사염화탄소에 녹지 않는다. [네이버 지식백과] 디에틸렌글리콜(다이에틸렌글리콜) [diethylene glycol, Diäthylenglykol] (화학대사전, 2001. 5. 20., 세화 편집부)용도
니트로디글리콜의 원료.용도
에틸렌옥사이드와 물 또는 에틸렌글리콜을 가열하든가, 에틸렌클로로히드린 또는 브로모히드린과 글리콜을 120℃에서 반응시킨다.화학적 성질
Diethylene glycol is a clear colorless, odorless and stable oily liquid. It is also slightly viscous, noncorrosive and nonvolatile. Because of its ether and alcohol group, diethylene glycol exhibits chemical properties characteristic of both primary alcohols and ethers. Its boiling point is considerably higher than that of ethylene glycol, and its solvent is greater. Diethylene glycol is miscible with water, ethers, lower aliphatic alcohols, aldehydes and ketones and is partially soluble in benzene, carbon tetrachloride, monobenzene, orthodichlorobenzene and toluene. It dissolves many dyes, resins, oils, nitrocellulose and many organic substances. Because of its solvent power, low volatility and hygroscopicity, it is used in textile lubricants, cutting oils, dry cleaning soap, printing inks, steam-set inks, and nongrain wood stains. In the textile industry diethylene glycol is used as a conditioning agent for wool, rayon, and cotton. As a solvent for dyes it makes a valuable assistant in dyeing and printing. The high hygroscopicity of diethylene glycol makes it an efficient softening agent for tobacco, paper, synthetic sponges, glues and casein. Diethylene glycol is especially useful in the dehydration of natural gas. A mixture of diethylene glycol and monoethanolamine will remove moisture, hydrogen sulfide and carbon dioxide from natural gas.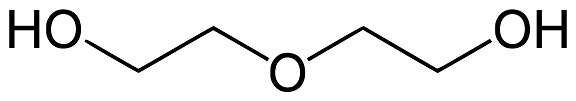
diethylene glycol structure
주요 응용
Diethylene glycol has many industrial uses. It is a component of antifreeze, brake fluids, cosmetics, inks, and drying agents, and it is used as a plasticizer. In antifreeze solution for sprinkler systems, water seals for gas tanks, etc. (water with 40% diethylene glycol freezes at -18°; with 50% at -28°); as lubricating and finishing agent for wool, worsted, cotton, rayon, and silk; as solvent for vat dyes; in composition corks, glues, gelatin, casein, and pastes to prevent drying out.생산 방법
Diethylene glycol is produced commercially as a by-product of ethylene glycol production. It can also be produced directly by reaction between ethylene glycol and ethylene oxide .일반 설명
Diethylene glycol appears as a colorless liquid. Denser than water. Contact may slightly irritate skin, eyes and mucous membranes. May be slightly toxic by ingestion. Used to make other chemicals.공기와 물의 반응
Slightly soluble in water.반응 프로필
Diethylene glycol is incompatible with strong oxidizing agents. Diethylene glycol is also incompatible with strong bases. Diethylene glycol can react with sulfuric acid and other dehydrating agents, nitric acid, oxygen, hydrogen peroxide, perchloric acid and strong acids. Mixtures with sodium hydroxide decompose exothermically when heated to 446° F.건강위험
Ingestion of large amounts may cause degeneration of kidney and liver and cause death. Liquid may cause slight skin irritation.화재위험
Diethylene glycol is combustible.Toxicology
The toxicity of diethylene glycol is similar to ethylene glycol and clearly is a CNS depressant. It has a low inhalation hazard because of its low vapor pressure; however, inhalation of the mist or aerosol is to be avoided. Workplace levels for vapors and aerosols cannot exceed 50 ppm. In case of accidental release of diethylene glycol, use of a full-face positive air pressure respirator is recommended. Even though the toxicokinetics in humans is not completely understood, its toxic nature is confirmed by animal studies. Several human cases were reported in the medical literature. Several children in Haiti died in 1995 and 1996 following the consumption of medication containing diethylene glycol. Similar other cases in children were reported in other countries as well. A 24-year-old man developed encephalopathy and rapidly became quadriplegic following ingestion of a solution containing diethylene glycol . Thus, the toxicity of diethylene glycol is well established.Safety Profile
Moderately toxic to humans by ingestion. Poison experimentally by inhalation. Moderately toxic by ingestion and intravenous routes. Questionable carcinogen with experimental carcinogenic,tumorigenic, and teratogenic data. An eye and human skin irritant. Combustible when exposed to heat or flame; can react with oxidning materials. To fight fire, use alcohol foam, water, Con, dry chemical. Mixtures with sodium hydroxide decompose exothermically when heated to 230℃ and release explosive hydrogen gas. When heated to decomposition it emits acrid smoke and irritating fumes. See also GLYCOL ETHERS.Carcinogenicity
Weil et al. , in their longterm studies on rats of three different age levels, found only one bladder tumor in those fed diets that contained 4% diethylene glycol. This tumor was in a rat that also had bladder stones . To clarify the question of the cause of the tumor, Weil et al. implanted calcium oxalate stones or glass beads into the bladders of rats. They found that bladder tumors never developed without the presence of a foreign body in the bladder. This led to the conclusion that diethylene glycol essentially free of ethylene glycol is not a primary carcinogen.환경귀착
Diethylene glycol is metabolized by alcohol dehydrogenase to toxic metabolites predominantly, HEAA and DGA. DEG can cause an anion gap metabolic acidosis, cortical necrosis resulting in permanent renal failure and neurotoxicity. DGA, not HEAA, was recently identified as being the primary nephrotoxic agent causing proximal tubule cell death. The neurotoxicity seen after DEG poisoning is only recently described. The neurotoxicity is delayed and has cranial and peripheral demyelinating sensorimotor polyneuropathy pattern. The exact mechanism of the neurotoxicity remains unclear and in the cases described in the literature, it appears to be prolonged but does show evidence of reversibility.다이에틸렌 글리콜 준비 용품 및 원자재
원자재
준비 용품
자일렌
polyurethane water-based emulsion finishes PU-II series
2,6-디클로로인도페놀 나트륨 염
2-클로로-4-도데실페놀
2,2'-OXYDIACETYL CHLORIDE
4-(2-THIENYL)BUTYRIC ACID
1-피렌부틸산
N-메틸모폴린
우르소데옥시콜산
4-아미노-2,6-디클로로페놀
Unsaturated polyester resin
디에틸렌글리콜모노노르말헥실에테르
polyurethae finishes PUC series
CSF series modified sacrylic binder
21-Iodo-16-methylpregna-1,4,9(11)-trien-17-ol-3,20-dione
AC anti-fungus leather finishing agent
톨루엔
디에틸렌글리콜디에틸에테르
4-(4-Methoxyphenyl)butyric acid
디에틸렌글리콜디벤조에이트
에틸디글리콜
6-Phenylhexanoic acid
디암페네타이드
thickening agent PAS
1-CHLORO-3-FLUOROISOPROPANOL
벤젠
16-Methylpregna-1,4,9(11)-trien-17-ol-3,20-dione
defoaming agent OTD
2,3-DIHYDROXYQUINOXALINE-6-CARBOXYLIC ACID
모노에틸렌글리콜
BT modified acrylic resin binder series
weather-proof acrylic binder series
1-PYRENEDECANOIC ACID
에틸모르폴린
카세인
4-벤질피페리딘
비스(2-부톡시에틸)에테르
1,3-DIFLUORO-2-PROPANOL
모르폴린
Disperse Yellow 126
다이에틸렌 글리콜 공급 업체
글로벌( 1154)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Aladdin Scientific | +1-+1(833)-552-7181 |
sales@aladdinsci.com | United States | 57511 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 |
sales@hbmojin.com | China | 12446 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 |
sales1@chuanghaibio.com | China | 5882 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 |
info@fdachem.com | China | 20293 | 58 |
| Hebei Saisier Technology Co., LTD | +86-18400010335 +86-18034520335 |
admin@hbsaisier.cn | China | 1009 | 58 |
| Hebei Zhuanglai Chemical Trading Co.,Ltd | +8613343047651 |
admin@zlchemi.com | China | 2999 | 58 |
| Hebei Andu Technology Com.,Ltd | +86-86-17798073498 +8617798073498 |
admin@hbandu.com | China | 300 | 58 |
| HebeiShuoshengImportandExportco.,Ltd | +86-18532138899 +86-18532138899 |
L18532138899@163.com | China | 931 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 |
sales@capotchem.com | China | 29798 | 60 |
| Shanghai Bojing Chemical Co.,Ltd. | +86-86-02137122233 +8613795318958 |
bj1@bj-chem.com | China | 299 | 55 |







