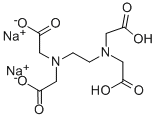
에틸렌디아민테트라아세트산, 이나트륨 염
|
|
에틸렌디아민테트라아세트산, 이나트륨 염 속성
- 녹는점
- 248 °C (dec.)(lit.)
- 끓는 점
- >100 °C
- 밀도
- 1.01 g/mL at 25 °C
- 증기압
- 0Pa at 25℃
- 저장 조건
- 2-8°C
- 용해도
- H2O: 투명, 무색
- 물리적 상태
- 액체
- 색상
- ≤5 (0.5 M)(APHA)
- 냄새
- 100.00%. 냄새 없는
- 수용성
- 물과 섞일 수 있습니다.
- BRN
- 3822669
- 안정성
- 흡습성
- LogP
- -4.3 at 25℃
- CAS 데이터베이스
- 139-33-3(CAS DataBase Reference)
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xn,C | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 36/38-36/37/38-22-34-21/22 | ||
| 안전지침서 | 26-36-37/39-45-36/37/39-36/37 | ||
| WGK 독일 | 2 | ||
| RTECS 번호 | AH4410000 | ||
| TSCA | Yes | ||
| HS 번호 | 29224999 | ||
| 유해 물질 데이터 | 139-33-3(Hazardous Substances Data) | ||
| 독성 | mouse,LD50,intraperitoneal,260mg/kg (260mg/kg),Nippon Yakurigaku Zasshi. Japanese Journal of Pharmacology. Vol. 52, Pg. 126S, 1956. | ||
| 기존화학 물질 | KE-13651 |
에틸렌디아민테트라아세트산, 이나트륨 염 C화학적 특성, 용도, 생산
용도
비누 / 세제 : 칼슘 및 마그네슘 비누 형성의 예방; 비누와 세제의 세제를 개선합니다. 과 붕산염과과 탄산염 표백제를 안정화시킵니다.화학 세척 : 옥살산 칼슘, 황산 칼슘, 탄산 칼슘, 황산 바륨, 및 공정 설비로부터의 다른 타입의 스케일을 제거한다.
I & I 청소기 : 스케일을 용해 스케일 형성을 방지, 계면 활성제의 세정력을 향상시킨다. 농축 된 액체 세제 및 샴푸의 탁도를 방지합니다. 유제, 윤활제 및 광택제에 대한 다가 금속 이온의 효과를 최소화합니다.
금속 마감 : 금속 제품의 산화막의 제거; 알칼리성 파쇄; 침산 조의 정전기 방지제; 알칼리 탈지.
섬유가 : 정련에 금속 이온을 제어, 종기 오프, 표백, 염색, 제거하고 작업을 마무리합니다. 형광 증 백제를 금속 촉매 산화 및 분해로부터 보호합니다. 염료의 변색을 방지합니다.
펄프 / 종이 : 수력 표백제의 효율성을 향상; 금속 촉매 밝기 복귀를 감소시킵니다. 스케일 제거 공정 장비에 사용됩니다. 염료가 금속으로 침전되는 것을 방지합니다.
순도시험
(1) 액성 : 이 품목의 수용액(1→100)의 pH는 4.3~4.7이어야 한다.
(2) 비소 : 이 품목을 비소시험법에 따라 시험할 때, 그 양은 4.0ppm 이하이어야 한다.
(3) 납 : 이 품목 5.0g을 취하여 원자흡광광도법 또는 유도결합플라즈마발광광도법에 따라 시험할 때, 그 양은 2.0ppm 이하이어야 한다.
(4) 시안화물 : 이 품목 1g을 환저플라스크에 취하여 물 100mL를 가하여 녹이고 인산 10mL를 가하여 증류한다. 수기에는 미리 0.5N 수산화나트륨용액 15mL를 넣은 100mL 메스플라스크를 사용하여 냉각기의 끝이 잠기게 하여 유액 100mL를 받아 시험용액으로 한다. 시험용액 2mL를 공전시험관에 취하여 페놀프탈레인시액 1방울을 가하여 묽은초산으로 중화하여 여기에 pH 6.8의 인산염완충액 5mL 및 클로라민용액(1→5) 1mL를 가해 바로 마개를 하고 조용히 혼화하여 2~3분간 방치한 다음 피리딘․피라졸론시액 5mL를 가하여 20~30℃에서 50분간 방치할 때 액의 색은 비교액보다 진하여서는 아니 된다. 비교액은 시안표준용액 1mL를 정확히 취하여 0.5N 수산화나트륨용액 15mL 및 물을 가하여 100mL로 하여 이 액 2mL를 공전시험관에 취하여 이하 시험용액과 같은 조작을 한다.
확인시험
(1) 이 품목의 수용액(1→20)은 확인시험법 중 (1) 나트륨염의 (나)의 반응을 나타낸다.
(2) 시험관에 물 5mL, 치오시안산암모늄시액 2방울 및 염화제이철시액 2방울을 가하여 얻어진 선홍색 용액에 이 품목 약 50mg을 가하여 섞으면 적색은 없어진다.
정량법
이 품목 0.4g을 정밀히 달아 물 20mL를 가하여 녹인 다음 암모니아․염화암모늄완충액(pH 10.7) 10mL를 가하여 0.05M 염화아연용액으로 적정한다(지시약 : 에리오크롬블랙T시액 2방울). 종말점은 액의 청색이 적색으로 변하는 점으로 한다.
0.05M 염화아연용액 1mL = 18.612mg C10H14N2Na2O8•2H2O
화학적 성질
Disodium edetate occurs as a white crystalline, odorless powder with a slightly acidic taste.용도
Ethylenediaminetetraacetic acid disodium salt is widely used in textile industry. Usually applied to dissolve limescale. It is applied in textile industry, pulp and paper industry and also in chelation therapy. In cosmetics, it acts as a sequestering agent. It acts as a corrosion inhibitor to carbon steel in the industries. It also acts as a food additive.주요 응용
Ethylenediaminetetraacetic acid disodium salt dihydrate has been in seed germination trials of plant species and in protein extraction from Moss, Physcomitrella paten. It has also been used in lysis and vacuole buffer for the isolation of vacuoles from Petunia petals.Chelator of divalent cations. Inhibits enzymes, such as metalloproteases, that require divalent cations for activity.
생산 방법
Disodium edetate may be prepared by the reaction of edetic acid and sodium hydroxide.정의
ChEBI: An organic sodium salt that is the anhydrous form of the disodium salt of ethylenediaminetetraacetic acid (EDTA).일반 설명
Ethylenediamine tetraacetate (EDTA) is a calcium ion chelator, that has a low molecular mass of 292.24 Da.Pharmaceutical Applications
Disodium edetate is used as a chelating agent in a wide range of pharmaceutical preparations, including mouthwashes, ophthalmic preparations, and topical preparations, typically at concentrations between 0.005 and 0.1% w/v.Disodium edetate forms stable water-soluble complexes (chelates) with alkaline earth and heavy-metal ions. The chelated form has few of the properties of the free ion, and for this reason chelating agents are often described as ‘removing’ ions from solution, a process known as sequestering. The stability of the metal–edetate complex is dependent on the metal ion involved and the pH.
Disodium edetate is also used as a water softener as it will chelate calcium and magnesium ions present in hard water. It is also used therapeutically as an anticoagulant as it will chelate calcium and prevent the coagulation of blood in vitro. Concentrations of 0.1% w/v are used in small volumes for hematological testing and 0.3% w/v in transfusions.
Safety Profile
Poison by intraperitoneal and intravenous routes. Moderately toxic by ingestion. Experimental teratogenic and reproductive effects. Mutation data reported. The calcium disodium salt of EDTA is used as a chelating agent in treating lead poisoning. When heated to decomposition it emits toxic fumes of NOx and NasO.저장
Edetate salts are more stable than edetic acid (see also Edetic acid). However, disodium edetate dihydrate loses water of crystallization when heated to 120°C. Aqueous solutions of disodium edetate may be sterilized by autoclaving, and should be stored in an alkali-free container.Disodium edetate is hygroscopic and is unstable when exposed to moisture. It should be stored in a well-closed container in a cool, dry place.
비 호환성
Disodium edetate behaves as a weak acid, displacing carbon dioxide from carbonates and reacting with metals to form hydrogen. It is incompatible with strong oxidizing agents, strong bases, metal ions, and metal alloys.Regulatory Status
GRAS listed. Included in the FDA Inactive Ingredients Database (inhalations; injections; ophthalmic preparations; oral capsules, solutions, suspensions, syrups, and tablets; rectal topical, and vaginal preparations). Included in nonparenteral and parenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.에틸렌디아민테트라아세트산, 이나트륨 염 준비 용품 및 원자재
원자재
준비 용품
에틸렌디아민테트라아세트산, 이나트륨 염 공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Qingdao Trust Agri Chemical Co.,Ltd | +8613573296305 |
aroma@qdtrustagri.com | China | 165 | 58 |
| BLiT (Hefei)Chemical Co.,Ltd | +86-551-62622640 |
sales@blitchem.com | China | 291 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 |
deasea125996@gmail.com | China | 2503 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 |
info@fdachem.com | China | 7845 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +8618740459177 |
sarah@tnjone.com | China | 893 | 58 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 |
fandachem@gmail.com | China | 9348 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 |
info@tnjchem.com | China | 2989 | 55 |
| career henan chemical co | +86-0371-86658258 |
sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Jinan Finer Chemical Co., Ltd | +86-531-88989536 +86-15508631887 |
sales@finerchem.com | China | 2967 | 58 |