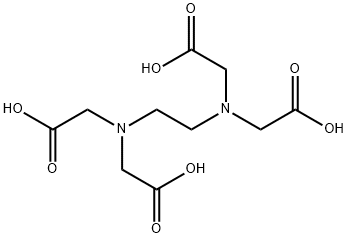
에틸렌다이아민테트라아세트산
|
|
에틸렌다이아민테트라아세트산 속성
- 녹는점
- 250 °C (dec.) (lit.)
- 끓는 점
- 434.18°C (rough estimate)
- 밀도
- 1.46 g/cm3 at 20 °C
- 증기압
- <0.013 hPa (20 °C)
- 굴절률
- n
20/D 1.363
- 인화점
- >400°C DIN 51758
- 저장 조건
- 2-8°C
- 용해도
- 3 M NaOH: 100mg/mL
- 산도 계수 (pKa)
- pKa 2 (Uncertain);10.26 (Uncertain)
- 물리적 상태
- 수정 같은
- 색상
- 흰색에서 거의 흰색
- 냄새
- 냄새 없는
- pH 범위
- 2.5 at 10 g/l at 23 °C
- 수소이온지수(pH)
- 2.5 (10g/l, H2O, 23℃)(slurry)
- 수용성
- 0.5g/L(25℃)
- 최대 파장(λmax)
- λ: 280 nm Amax: ≤0.25
- 분해온도
- 240 °C
- Merck
- 14,3517
- BRN
- 1716295
- 안정성
- 안정적인. 구리, 구리 합금, 니켈, 알루미늄, 강산화제, 강염기와 호환되지 않습니다.
- LogP
- -0.836 (est)
- CAS 데이터베이스
- 60-00-4(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xi | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 36-52/53-36/37/38-36/38 | ||
| 안전지침서 | 26-61-37/39-36 | ||
| 유엔번호(UN No.) | UN 3077 9 / PGIII | ||
| WGK 독일 | 2 | ||
| RTECS 번호 | AH4025000 | ||
| F 고인화성물질 | 3 | ||
| 자연 발화 온도 | >200 °C | ||
| TSCA | Yes | ||
| HS 번호 | 2922 49 85 | ||
| 유해 물질 데이터 | 60-00-4(Hazardous Substances Data) | ||
| 독성 | LD50 orally in Rabbit: 2580 mg/kg | ||
| 기존화학 물질 | KE-13648 |
에틸렌다이아민테트라아세트산 C화학적 특성, 용도, 생산
물성
냉수, 알코올 및 일반 유기 용제에는 녹지 않으며 뜨거운 물에는 약간 용해되지만 수산화 나트륨, 탄산나트륨 및 암모니아 용액에는 용해되는 백색 결정 분말 융점 240℃(분해).개요
EDTA는 칼슘과 같은 다양한 다가 양이온을 격리하는 킬레이트 제입니다. 그것은 제약 제조 및 식품 첨가제로 사용됩니다.주로 금속 이온 complexing 및 금속 분리에 적용; 수처리제 및 탈지제로 사용되는 첨가제; 물에서 금속 이온으로 인한 생산에 대한 유해한 영향을 방지 할 수있는 것; 사진, 제지 및 오일 필드 용 화학 물질. 화학 분석에서 보더 샌드 시약을 청소할 때도 사용됩니다.용도
EDTA는 욕조세제의 첨가물이기도 한데, 이는 EDTA가 비누의 음이온 성분과 반응하여 욕조에 끼는 때의 주성분인 침전을 형성하는 Ca2+이온과 반응하기 때문에 침전의 용해도를 증가시켜 때가 씻겨질 수 있게 하기 때문이다. EDTA는 물 속에 들어있는 금속 이온을 제거하는 데도 이용된다. EDTA는 Pb2+이온과 강한 친화성을 가지기 때문에 납중독의 치료제로도 사용되어 왔다. EDTA와 철 이온의 착물은 식물에 철 성분을 공급하는 데 이용되기도 한다. EDTA로 식용유에 존재하는 산화반응의 촉매인 금속 이온을 제거하여 식용유가 썩는 것을 방지할 수 있다. 생화학이나 분자생물학에서는 DNA나 단백질에 관련된 실험을 할 때, 시료가 효소로 인하여 손상되는 것을 막기 위해 효소가 필요로 하는 금속 이온을 제거할 목적으로 EDTA를 사용한다. EDTA는 혈액의 응고방지제로도 이용된다. 분석화학에서는 EDTA를 주로 금속 이온의 분석, 분리, 제거 등에 활용한다. EDTA는 완충용액의 성분으로 사용될 수 있다.개요
Ethylenediaminetetraacetic Acid (EDTA) is a common polydentate ligand. In EDTA, the hydrogen atoms are easily removed in solution to produce anionic EDTA4-. In its anionic form Ethylenediaminetetraacetic Acid (EDTA) has six binding atoms, two nitrogen and four oxygen.Ethylenediaminetetraacetic Acid (EDTA) binds to a metal ion at the six binding sites, wrapping itself around the metal ion, forming a very stable complex.the strong grasp of Ethylenediaminetetraacetic Acid (EDTA) on the metal ion is analogous to a crab or lobster clamping down on an object with its claw, hence the name chelation. Ethylenediaminetetraacetic Acid (EDTA) is such an effective chelating agent because it can deactivate a metal at up to six sites.
화학적 성질
Ethylenediaminetetraacetic acid is a solid.역사
Ethylenediaminetetraacetic Acid (EDTA) was first synthesized in the early 1930s by the German chemist Ferdinand Münz working for I. G. Farben. Münz, who was looking for a substitute for citric acid to use with dye solutions in the textile industry, was the first to patent a process for Ethylenediaminetetraacetic Acid (EDTA) synthesis in Germany in 1935. Münz subsequently applied for United States patents in 1936 and 1937 (U.S. Patent Number 2130505); his method involved reacting monochloroacetic acid (C2H3ClO2) and ethylene diamine (C2H8N2). Concurrent with Münz’s work, Frederick C. Bersworth in the United States synthesized Ethylenediaminetetraacetic Acid (EDTA) using different methods that gave greater yields and made EDTA’s commercial production economically viable. Bersworth syntheses involved reacting formaldehyde, amines, and hydrogen cyanide. Bersworth and Münz obtained patents for Ethylenediaminetetraacetic Acid (EDTA) production in the 1940s (U.S. Patent Numbers 2407645 and 2461519).용도
antispasmodic생산 방법
Edetic acid may be prepared by the condensation of ethylenediamine with sodium monochloroacetate in the presence of sodium carbonate. An aqueous solution of the reactants is heated to about 90°C for 10 hours, then cooled, and hydrochloric acid is added to precipitate the edetic acid.Edetic acid may also be prepared by the reaction of ethylenediamine with hydrogen cyanide and formaldehyde with subsequent hydrolysis of the tetranitrile, or under alkaline conditions with continuous extraction of ammonia.
정의
An organic chelating agent.일반 설명
Ethylenediamine tetraacetic acid is a colorless crystalline solid. Ethylenediaminetetraacetic acid is slightly soluble in water. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. Ethylenediaminetetraacetic acid is used in chemical analysis, to make detergents and cleaning compounds, and for many other uses.공기와 물의 반응
Slightly soluble in water.농업용
EDTA is short for ethylenediamhetetraacetic acid, an amino polycarboxylic acid. It is a tetraprotic acid and is represented as H4Y with four carboxyl groups and two nitrogen atoms acting as ligand sites. Thus the compound is a hexadentate ligand. Ligands include ions such as Cl-, NO2-and CN- or neutral molecules like NH3 and H2O, which possess a lone pair of electrons that can be shared with a metal cation in coordinate covalent bonds.The water solubility of EDTA is very low and, therefore, its di-sodium salt Na2H2Y.2H2O is commonly used in titrations. The Y4- forms very stable, one-to-one complexes with practically every metal ion in the Periodic Table. The reactions are carried out in a neutral or alkaline medium as the complex decomposes in acidic medium.
(and hence deterioration) of the food product, (d) to increase the storage life of whole blood by removing free calcium ions (Ca2+) to inhibit clotting, and (e) for extracting trace elements, especially copper. EDTA metal complexes, such as NaFeEDTA, MnEDTA, ZnEDTA and CuEDTA are used as fertilizers and foliar sprays.
생물학적 활성
Chelating agent; sequesters di- and trivalent metal ions.Safety Profile
Poison by intraperitoneal route. Experimental teratogenic and reproductive effects. Mutation data reported. A general-purpose chelaung and complexing agent. When heated to decomposition it emits toxic fumes of NOx.Safety
Edetic acid and edetates are widely used in topical, oral, and parenteral pharmaceutical formulations. They are also extensively used in cosmetics and food products.Edetic acid is generally regarded as an essentially nontoxic and nonirritant material, although it has been associated with doserelated bronchoconstriction when used as a preservative in nebulizer solutions. It has therefore been recommended that nebulizer solutions for bronchodilation should not contain edetic acid.
Edetates, particularly disodium edetate and edetate calcium disodium, are used in a greater number and variety of pharmaceutical formulations than the free acid.
Disodium edetate, trisodium edetate, and edetic acid readily chelate calcium and can, in large doses, cause calcium depletion (hypocalcemia) if used over an extended period or if administered too rapidly by intravenous infusion. If used in preparations for the mouth, they can also leach calcium from the teeth. In contrast, edetate calcium disodium does not chelate calcium. Edetate calcium disodium is nephrotoxic and should be used with caution in patients with renal impairment.
The WHO has set an estimated acceptable daily intake for disodium edetate in foodstuffs at up to 2.5 mg/kg body-weight.
LD50 (mouse, IP): 0.25 g/kg
LD50 (rat, IP): 0.397 g/kg
잠재적 노출
EDTA is a white, odorless, crystalline material or white powder환경귀착
EDTA can be very persistent in water, including wastewatertreatment plants. EDTA is often found in the receiving waters of many industrial areas, thus being classified as one of the major organic pollutants discharged in waters. The available ecotoxicity data for EDTA indicate that these compounds are slow to degrade under typical environmental conditions but are not expected to bioconcentrate. EDTA compounds range from practically nontoxic to moderately toxic on an acute basis, depending on the salt. Algae and invertebrates are among the most sensitive species based on predictive modeling for acute and chronic endpoints for EDTA, depending on the compound. EDTA and its salts also do not appear to be very toxic for terrestrial wild mammals, and adverse effects from reasonably expected agricultural uses are not expected.저장
Although edetic acid is fairly stable in the solid state, edetate salts are more stable than the free acid, which decarboxylates if heated above 150°C. Disodium edetate dihydrate loses water of crystallization when heated to 120°C. Edetate calcium disodium is slightly hygroscopic and should be protected from moisture.Aqueous solutions of edetic acid or edetate salts may be sterilized by autoclaving, and should be stored in an alkali-free container.
Edetic acid and edetates should be stored in well-closed containers in a cool, dry place.
운송 방법
UN3082 Environmentally hazardous substances, liquid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name RequiredPurification Methods
Dissolve EDTA in aqueous KOH or ammonium hydroxide, and precipitate it twice with dilute HCl or HNO3. Boil it twice with distilled water to remove mineral acid, then recrystallise it from water or dimethylformamide. Dry it at 110o. It also recrystallises from boiling 1N HCl; wash the crystals with distilled H2O and dry them in vacuo. [Ma & Ray Biochemistry 19 751 1980, Beilstein 4 IV 2449.]비 호환성
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, copper, copper alloys, and nickelRegulatory Status
Included in the FDA Inactive Ingredients Database (oral, otic, rectal, and topical preparations; submucosal injection preparations). Included in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.에틸렌다이아민테트라아세트산 준비 용품 및 원자재
원자재
Concentrated hydrochloric acid
에틸렌디아민
클로로아세트산
formaldehyde
페놀프탈레인
포르말린
Ethylenediamine aqueous solution
Cyanide, Quant Test Strips
에틸렌디아민테트라아세트산,테트라나트륨염(2수화물)
사이안화 나트륨
시안화수소
염산
Sodium edetate
메틸알콜
수산화나트륨
Activated carbon,decolor
글리콜산
황산
소듐 클로로아세테이트
준비 용품
에틸렌다이아민테트라아세트산 공급 업체
글로벌( 1155)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Jiangsu Boquan Biotechnology Co., Ltd. | +86-18168774353 |
boquanshengwu003@boquansw.com | China | 248 | 58 |
| DONBOO AMINO ACID COMPANY | +8613063595538 |
donboo@donboo.com | China | 9365 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 |
peter@yan-xi.com | China | 5873 | 58 |
| Shanghai UCHEM Inc. | +862156762820 +86-13564624040 |
sales@myuchem.com | China | 7193 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 |
sales1@chuanghaibio.com | China | 5882 | 58 |
| Hebei Dangtong Import and export Co LTD | +86-13910575315 +86-13910575315 |
admin@hbdangtong.com | China | 1005 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 |
deasea125996@gmail.com | China | 2503 | 58 |
| Hebei Kingfiner Technology Development Co.Ltd | +86-15532196582 +86-15373005021 |
lisa@kingfinertech.com | China | 2990 | 58 |
| Anhui Ruihan Technology Co., Ltd | +8617756083858 |
daisy@anhuiruihan.com | China | 994 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 |
info@fdachem.com | China | 20293 | 58 |