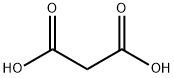
말론산
|
|
말론산 속성
- 녹는점
- 132-135 °C (dec.) (lit.)
- 끓는 점
- 140℃(decomposition)
- 밀도
- 1.619 g/cm3 at 25 °C
- 증기압
- 0-0.2Pa at 25℃
- 굴절률
- 1.4780
- 인화점
- 157°C
- 저장 조건
- Sealed in dry,Room Temperature
- 용해도
- 1 M NaOH: 가용성 100mg/mL, 투명하거나 약간 흐릿함, 무색 내지 희미한 노란색
- 산도 계수 (pKa)
- 2.83(at 25℃)
- 물리적 상태
- 액체
- 색상
- 하얀색
- 수소이온지수(pH)
- 3.17(1 mM solution);2.5(10 mM solution);1.94(100 mM solution)
- 수용성
- 1400g/L(20℃)
- Merck
- 14,5710
- BRN
- 1751370
- 안정성
- 산화제, 환원제, 염기와 호환되지 않습니다.
- InChIKey
- OFOBLEOULBTSOW-UHFFFAOYSA-N
- LogP
- -0.81
- CAS 데이터베이스
- 141-82-2(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xn,Xi | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 20/22-41-36/37/38-22 | ||
| 안전지침서 | 26-36/39-37/39-36 | ||
| 유엔번호(UN No.) | 3261 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | OO0175000 | ||
| TSCA | Yes | ||
| 포장분류 | III | ||
| HS 번호 | 29171910 | ||
| 유해 물질 데이터 | 141-82-2(Hazardous Substances Data) | ||
| 독성 | mouse,LD50,intraperitoneal,300mg/kg (300mg/kg),National Technical Information Service. Vol. AD277-689, | ||
| 기존화학 물질 | KE-23182 |
말론산 C화학적 특성, 용도, 생산
개요
Malonic acid (MA), also known as propanedioic acid, is a dicarboxylic acid with structure CH2(COOH)2. It have three kinds of crystal forms, of which two are triclinic, and one is monoclinic. That crystallized from ethanol is white triclinic crystals.It decomposes to acetic acid and carbon dioxide at 140℃. It does not decompose at 1.067×103~1.333×103Pa vacuum, but directly sublimates. The ionised form of malonic acid, as well as its esters and salts, are known as malonates. For example, diethyl malonate is malonic acid's ethyl ester. The name originates from Latin malum, meaning apple.화학적 성질
Malonic acid is a white crystalline solid that decomposes at approximately 135°C. It has high solubility in water and oxygenated solvents and exhibits greater acidity than acetic acid, which has a pK value of 4.75. The pKa values for the loss of its first and second protons are 2.83 and 5.69, respectively. It is slightly soluble in pyridine. It can decompose to formic acid and carbon dioxide in case of potassium permanganate. Since that malonic acid generates carbon dioxide and water after heated without pollution problems, it can be directly used as aluminum surface treatment agent.용도
Malonic acid is used as an intermediate in the manufacture of barbiturates and other pharmaceuticals. It is a component used as a stabilizer in many high-end cosmetic and pharmaceutical products. Malonic acid is also used as building block in chemical synthesis, specifically to introduce the molecular group -CH2-COOH. It is used for the introduction of an acetic acid moiety under mild conditions by Knoevenagel condensation and subsequent decarboxylation.주요 응용
Malonic acid is acts as a building block in organic synthesis. It is also useful as a precursor for polyesters and alkyd resins, which is used in coating applications, thereby protecting against UV light, corrosion and oxidation. It acts as a cross linker in the coating industry and surgical adhesive. It finds application in the production of specialty chemicals, flavors and fragrances, polymer cross linkers and pharmaceuticals.정의
ChEBI: Malonic acid is an alpha,omega-dicarboxylic acid in which the two carboxy groups are separated by a single methylene group. It has a role as a human metabolite. It is a conjugate acid of a malonate(1-).제조 방법
Malonic acid is usually produced from chloroacetic acid.Reaction: The chloroacetic acid is added to the reaction kettle by adding sodium carbonate aqueous solution to generate sodium chloroacetate aqueous solution, and then 30% sodium cyanide solution is slowly added dropwise, and the reaction is carried out at a predetermined temperature to generate sodium cyanoacetate. After the cyanation reaction is completed, add sodium hydroxide for heating and hydrolysis to generate sodium malonate solution, concentrate, then dropwise add sulfuric acid for acidification to generate malonic acid, filter and dry to obtain the product.
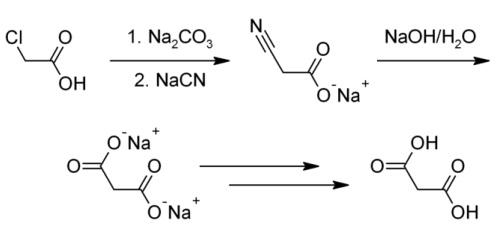
This method often does not produce a pure enough product or the pure product has an extremely low yield. Industrially, malonic acid is also produced by hydrolyzing dimethyl malonate or diethyl malonate. This manufacturing method is able to bring about a higher yield and purity, but the organic synthesis of malonic acid through these processes is extremely costly and environmentally hazardous.
화학 반응
In a well - known reaction, malonic acid condenses with urea to form barbituric acid. Malonic acid is also frequently used as an enolate in Knoevenagel condensations or condensed with acetone to form Meldrum's acid. The esters of malonic acid are also used as a - CH2COOH synthon in the malonic ester synthesis.Biological Functions
Malonic acid is the classic example of a competitive inhibitor of the enzyme succinate dehydrogenase (complex II), in the respiratory electron transport chain.It binds to the active site of the enzyme without reacting, competing with the usual substrate succinate but lacking the ?CH2CH2? group required for dehydrogenation. This observation was used to deduce the structure of the active site in succinate dehydrogenase.일반 설명
White crystals or crystalline powder. Sublimes in vacuum.공기와 물의 반응
Water soluble.위험도
Strong irritant.화재위험
Flash point data for Malonic acid are not available; however, Malonic acid is probably combustible.Biotechnological Applications
The calcium salt of malonic acid occurs in high concentrations in beetroot. It exists in its normal state as white crystals. Malonic acid is the classic example of a competitive inhibitor: It acts against succinate dehydrogenase (complex II) in the respiratory electron transport chain.Purification Methods
Crystallise malonic acid from *benzene/diethyl ether (1:1) containing 5% of pet ether (b 60-80o), wash with diethyl ether, then recrystallise it from H2O or acetone. Dry it under vacuum over conc H2SO4. [Beilstein 2 IV 1874.]말론산 준비 용품 및 원자재
원자재
준비 용품
4-(MORPHOLIN-4-YLMETHYL)-1,3-THIAZOL-2-AMINE
Diethyl ketomalonate
3-(3,4,5-트리메톡시페닐)프로피온산
TARTRONIC ACID
Methyl trans-2-nonenoate
2,4,5-트리메톡시신남산
4-CHLORO-BETA-METHYL-Y-OXO-BENZENEBUTANOIC ACID
하이드록시카프릴산락통
5,5-디메틸-1,3-사이클로헥사디온
3-PYRIDIN-2-YL-PROPIONIC ACID H2SO4
trans-Ferulic acid
trans-3-Hexenoic acid
4-BROMO-PYRAN-2-ONE
4-hydroxy-7-methyl-1,8-naphthyridine-3-carboxylic acid
6-METHOXY-2-OXO-2H-CHROMENE-3-CARBOXYLIC ACID
3-(트리플루오로메톡시)신남산
Diethyl butylmalonate
3,3-DIMETHOXYESTR-5(10)-ENE-17 B OL
3-AMINO-3-(2-THIENYL)PROPANOIC ACID
Diethyl(phenylacetyl)malonate
Antifreeze
DIETHYL SEC-BUTYLMALONATE
4-ETHOXYCINNAMIC ACID
3,5-DIMETHOXYCINNAMIC ACID
(1H-INDAZOL-3-YL)-ACETIC ACID
4-플루오로신나믹 산
3-(2-METHYLPHENYL)PROPIONIC ACID
5-BROMO-2-FLUOROCINNAMIC ACID
3-(3-CHLOROPHENYL)PROPIONIC ACID
3-(4-METHYLPHENYL)PROPIONIC ACID
Diethyl butyrylmalonate
3-Methoxycinnamic acid
Diethyl nitromalonate
3-Pyridinepropionic acid
3-(2-푸릴)아크릴산
3-(1-NAPHTHYL)-PROPIONIC ACID
2-Acetylbenzoic acid
3-(3-METHYL-2-THIENYL)ACRYLIC ACID
3-Pyridineacrylic acid
diethyl [(m-chloroanilino)methylene]malonate
말론산 공급 업체
글로벌( 826)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Shanghai Time Chemicals CO., Ltd. | +86-021-57951555 +8617317452075 |
jack.li@time-chemicals.com | China | 1807 | 55 |
| Kindchem(Nanjing)Co.,Ltd | +86-025-025-85281586 +8618651653755 |
sales@kindchem.cn | China | 1227 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 |
sales@tnjchem.com | China | 34572 | 58 |
| Wuhan Pinestone Fortune International Trading Co., Ltd. | +86-027-85615902 +86-13971435335 |
imp.exp8@fengfan.net | China | 29 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 |
info@fdachem.com | China | 7845 | 58 |
| Ouhuang Engineering Materials (Hubei) Co., Ltd | +8617702722807 |
admin@hbouhuang.com | China | 2259 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 |
sales@capotchem.com | China | 29797 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21691 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 |
ivan@atkchemical.com | China | 32480 | 60 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 |
info@tnjchem.com | China | 2989 | 55 |