- Minocycline
-
- $0.00 / 10mg
-
2024-09-20
- CAS:10118-90-8
- Min. Order: 10mg
- Purity: 0.98
- Supply Ability: 5g
- Minocycline
-

- $100.00 / 1KG
-
2023-08-31
- CAS:10118-90-8
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 5000KG
- Minocycline
-

- $10.00 / 1kg
-
2023-07-31
- CAS:10118-90-8
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 50000 Pieces/Year
Related articles - What is Minocycline?
- Minocycline is mainly marketed for oral administration, but a more soluble formulation of minocycline is available in some cou....
- Mar 17,2022
|
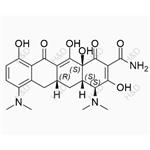
| Product Name: | Minocycline | | Synonyms: | 7-DIMETHYLAMINO-6-DEMETHYL-6-DEOXYTETRACYCLINE HYDROCHLORIDE;7-DIMETHYLAMINO-6-DEMETHYL-6-DEOXYTETRACYCLINE, HCL;MYNOCINE;MINOCIN;MINOCYCLINE;MinocyclineBase;Minocycline Hcl 13614-98-7 / Base;2-Naphthacenecarboxamide, 4,7-bis(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,10,12,12a-tetrahydroxy-1,11-dioxo- (8CI) | | CAS: | 10118-90-8 | | MF: | C23H27N3O7 | | MW: | 457.48 | | EINECS: | 800-277-5 | | Product Categories: | | | Mol File: | 10118-90-8.mol | 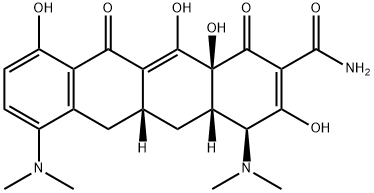 |
| | Minocycline Chemical Properties |
| alpha | D25 -166° (c = 0.524) | | Boiling point | 563.31°C (rough estimate) | | density | 1.3283 (rough estimate) | | refractive index | 1.6500 (estimate) | | solubility | Methanol (Slightly) | | pka | pKa 2.8 (Uncertain);5.0 (Uncertain);7.8 (Uncertain);9.5 (Uncertain) | | form | Solid | | color | Yellow to Dark Orange | | Water Solubility | 52g/L(25 ºC) | | Stability: | Light Sensitive | | CAS DataBase Reference | 10118-90-8(CAS DataBase Reference) | | EPA Substance Registry System | Minocycline (10118-90-8) |
| | Minocycline Usage And Synthesis |
| Description | Minocycline belongs to the class of medications called tetracycline antibiotics, who has a broader spectrum than the other members of the group. Identified as a long-acting type, it is used to treat infections induced by certain kinds of bacteria. Minocycline is commonly used in the treatment of skin infections, such as inflammatory lesions of non-nodular moderate-to-severe acne vulgaris. It is also used for other skin infections, such as MRSA and Lyme disease. Besides, minocycline is effective to treat urinary tract infections, gallbladder infections, and respiratory tract infections like bronchitis, pneumonia, sinusitis and it is also used for treating Rocky Mountain spotted fever, typhus and other infections caused by the typhus group of bacteria and tick fevers caused by rickettsiae, etc.
Patented in 1961, minocycline was put into commercial use in 1971. It is not a naturally occurring antibiotic, but was synthesized semi-synthetically from natural tetracycline antibiotics by Lederle Laboratories in 1966.
| | References | https://en.wikipedia.org/wiki/Minocycline
http://www.medicinenet.com/minocycline-oral/article.htm
http://bodyandhealth.canada.com/drug/getdrug/ratio-minocycline
| | Description | An important antibiotic produced by semisynthesis from demeclocycline is minocycline. It is much more
lipophilic than its precursors, gives excellent blood levels following oral administration (90–100% available),and can be given once a day. Its absorption is lowered by approximately 20% when taken with food or milk.
It is less dependent on active uptake mechanisms and has a somewhat broader antimicrobial spectrum. It
also, apparently, is less painful on IM or IV injection, but it has vestibular toxicities (e.g., vertigo, ataxia, and
nausea) not generally shared by other tetracyclines. | | Originator | Minocin,Lederle ,US,1971 | | Uses | Minocycline is used for the same indications as other antibiotics of the tetracycline series.
In a few cases, it is tolerated worse than other tetracyclines, and in particular, it has an
effect on the vestibular apparatus. In addition, as seen already from the synthesis scheme,
it is much more expensive than other tetracyclines, which are synthesized in a purely
microbiological manner. Synonyms of this drug are clinocin, minocyn, vectrin, and others. | | Uses | Minocycline is a semi-synthetic tetracycline prepared by sequential hydrogenolysis, nitration and reductive methylation. Minocycline, together with doxycycline, is regarded as a ‘third generation’ tetracycline largely replacing the natural products and pro-drugs produced in the early 1950s for mainstream antibiotic applications. Like all tetracyclines, minocycline shows broad spectrum antibacterial and antiprotozoan activity and acts by binding to the 30S and 50S ribosomal sub-units, blocking protein synthesis. Minocycline has been extensively cited in the literature with over 5,000 references. | | Definition | ChEBI: A tetracycline analogue having a dimethylamino group at position 7 and lacking the methyl and hydroxy groups at position 5. | | Indications | The tetracycline antibiotic minocycline (Minocin) is
modestly effective in the treatment of rheumatoid
arthritis and is generally well tolerated. Radiographic
evidence of its efficacy as a DMARD is lacking, although
clinical symptoms do abate. It can be useful in
the treatment of early, mild disease. | | Manufacturing Process | Preparation of 7-(N,N'-Dicarbobenzyloxyhydrazino)-6-Demethyltetracycline: A1.0 g portion of 6-demethyltetracycline was dissolved in a mixture of 9.6 ml oftetrahydrofuran and 10.4 ml of methanesulfonic acid at -10°C. The mixturewas allowed to warm to 0°C. A solution of 0.86 g of dibenzyl azodicarboxylatein 0.5 ml of tetrahydrofuran was added dropwise and the mixture was stirredfor 2 hours while the temperature was maintained at 0°C. The reactionmixture was added to ether. The product was filtered off, washed with etherand then dried. The 7-(N,N'-dicarbobenzyloxyhydrazino)-6-demethyltetracycline was identified by paper chromatography.
Reductive Methylation of 7-(N,N'-Dicarbobenzyloxyhydrazino)-6-Demethyl-6-Deoxytetracycline to 7-Dimethylamino-6-Demethyl-6-Deoxytetracycline: Asolution of 100 mg of 7(N,N'-dicarbobenzyloxyhydrazino)-6-demethyl-6-deoxytetracycline in 2.6 ml of methanol, 0.4 ml of 40% aqueous ormaldehyde solution and 50 mg of 5% palladium on carbon catalyst washydrogenated at room temperature and two atmospheres pressure. Uptake ofthe hydrogen was complete in 3 hours. The catalyst was filtered off and thesolution was taken to dryness under reduced pressure. The residue wastriturated with ether and then identified as 7-dimethylamino-6-demethyl-6-deoxytetracycline by comparison with an authentic sample, according to USPatent 3,483,251. | | Brand name | Dynacin (Medicis); Minocin (Lederle); Minocin (Triax);
Solodyn (Medicis);Klinomycin;Lederderm;Mino-50;Minomycin. | | Therapeutic Function | Antibiotic | | World Health Organization (WHO) | Minocycline, a semi-synthetic tetracycline derivative was
introduced in 1967. It is used today in the treatment of bacterial, rickettsial and
amoebic infections. Symptoms described as dizziness or vertigo have been
recognized in association with minocycline administration, however, these
symptoms are usually not severe. Minocycline is registered in many countries and
the World Health Organization is not aware that registration has been refused
elsewhere. | | Antimicrobial activity | It exhibits the broad-spectrum activity
typical of the group, but retains activity against some strains
of Staph. aureus resistant to older tetracyclines. It is active
against β-hemolytic streptococci and some tetracycline-
resistant
pneumococci. It is also active against some enterobacteria
resistant to other tetracyclines, probably because
some Gram-negative efflux pumps remove minocycline less
effectively
than other tetracyclines. Some strains of H. influenzae resistant
to other tetracyclines are susceptible. Sten. maltophilia
is susceptible, as are most strains of Acinetobacter spp.
and L. pneumophila.
It is notable for its activity against Bacteroides and
Fusobacterium spp., and is more active than other tetracyclines
against C. trachomatis, brucellae and nocardiae. It inhibits
Mycobacterium tuberculosis, M. bovis, M. kansasii and M. intracellulare
at 5–6 mg/L. Candida albicans and C. tropicalis are also
slightly susceptible. | | Pharmaceutical Applications | A semisynthetic tetracycline derivative supplied as the hydrochloride
for oral administration. | | Pharmacokinetics | Oral absorption: 95–100%
Cmax 150 mg oral: 4 mg/L after 2h
300 mg oral: 2 mg/L after 2 h
Plasma half-life: 12–24 h
Volume of distribution: 80–115 L
Plasma protein binding: 76%
Absorption
Food does not significantly affect absorption, which is depressed
by co-administration with milk. It is chelated by metals and
suffers the effects of antacids and ferrous sulfate common to
tetracyclines. On a regimen of 100 mg every 12 h, steady-state
concentrations ranged between 2.3 and 3.5 mg/L.
Distribution
The high lipophilicity of minocycline provides wide distribution
and tissue concentrations that often exceed those of
the plasma. The tissue:plasma ratio in maxillary sinus and
tonsillar tissue is 1.6: that in lung is 3–4. Sputum concentrations
may reach 37–60% of simultaneous plasma levels.
In bile, liver and gallbladder the ratios are 38, 12 and 6.5,
respectively.
Prostatic and seminal fluid concentrations range from 40%
to 100% of those of serum. CSF penetration is poor, especially
in the non-inflamed state. Concentrations in tears and
saliva are high, and may explain its beneficial effect in the
treatment of meningococcal carriage.
Metabolism
Biotransformation to three microbiologically inactive
metabolites occurs in the liver: the most abundant is
9-hydroxyminocycline.
Excretion
Only 4–9% of administered drug is excreted in the urine, and
in renal failure elimination is little affected. Neither hemodialysis
nor peritoneal dialysis affects drug elimination. Fecal excretion is relatively low and evidence for enterohepatic
recirculation remains uncertain. Despite high hepatic excretion,
dose accumulation does not occur in liver disease, such
as cirrhosis. Type IIa and type IV hyperlipidemic patients
show a decreased minocycline clearance of 50%, suggesting
that dose modification may be necessary. | | Clinical Use | There appear to be few situations in which it has a unique
therapeutic advantage over other tetracyclines. Its use has been
tempered by the high incidence of vestibular side effects.
Although used in the long-term management of acne, the
potential for skin pigmentation must be considered. Because
of its high tissue concentrations, it may occasionally provide a
useful alternative to other agents for the treatment of chronic
prostatitis. It has a role in the treatment of sexually transmitted
chlamydial infections. | | Side effects | Minocycline shares the untoward reactions common to the
group with gastrointestinal side effects being most common,
and more prevalent in women. Diarrhea is relatively
uncommon, presumably as a result of its lower fecal concentrations.
Hypersensitivity reactions, including rashes,
interstitial nephritis and pulmonary eosinophilia, are occasionally
seen.
Staining of the permanent dentition occurs with all tetracyclines;
a side effect that appears to be unique to minocycline
is that of tissue discoloration and skin pigmentation. Tissues
that may become pigmented include the skin, skull and other
bones and the thyroid gland, which at autopsy appears blackened.
The pigmentation tends to resolve slowly with discontinuation
of the drug and is related to the length of therapy.
Three types of pigmentation have been identified:
? A brown macular discoloration (‘muddy skin syndrome’),
which occurs in sun-exposed parts and is histologically
associated with melanin deposition.
? Blue–black macular pigmentation occurring within
inflamed areas and scars associated with hemosiderin
deposition.
? Circumscribed macular blue–gray pigmented areas
occurring in sun-exposed and unexposed skin, which
appears to be linked to a breakdown product of
minocycline.
CNS toxicity has been prominent, notably benign intracranial
hypertension, which resolves on discontinuation of the
drug, and, more commonly, dizziness, ataxia, vertigo, tinnitus,
nausea and vomiting, which appear to be more frequent
in women. These primarily vestibular side effects have ranged
in frequency from 4.5% to 86%. They partly coincide with
plasma concentration peaks, but their exact pathogenesis has
yet to be determined. | | Synthesis | Minocycline, 4,7-bis(dimethylamino)-1,4,4a,5,5a,6,11,12 a-octahydro-
3,10,12,12a-tetrahydroxy-1,11-dioxo-2-naphthacencarboxamide (32.3.17), is synthesized
from 6-dimethyl-tetracycline (32.3.11), which is synthesized as a result of the vital activity
of S. aureofaciens, in which the mechanism of transferring methyl groups is disrupted, or
from a common strain of the same microorganisms, but with the addition of compounds
such as ethionin, D-norleucine or D-methionine to the medium for developing this actino�mycete, which are antimetabolytes of methionine, the primary donor of methyl groups in
microbiological synthesis of tetracycline molecules. Hydrogenolysis of the aforementioned
6-demethyltetracycline (32.3.11) with hydrogen using a palladium on carbon catalyst gives
4-dimethylamino-1,4,4a,5,5a,6,11,12a-octahydro-3,10,12,12a-tetrahydroxy-1,11-dioxo-
2-napthacencarboxamide (32.3.12), which is nitrated at position 9 by potassium nitrate in
aqueous hydrofluoric acid, which forms the nitro compound (32.3.13). This is reduced to
the corresponding amino derivative (32.3.14) by hydrogen over platinum dioxide. The
resulting aminophenyl compound (32.3.14) is then nitrated with nitric acid in the presence
of sulfuric acid to make 7-nitro-9-amino-4-naphthacencarboxamide (32.3.15).
This under�goes diazotization when reacted with butylnitrate in sulfuric acid, and the resulting diazo
derivative (32.3.16) is reduced with hydrogen using a palladium on carbon catalyst. During
this, the product is deazotized, while the nitro group is simultaneously reduced to an amino
group, which undergoes exhaustive methylation by formaldehyde into minocycline
(32.3.17).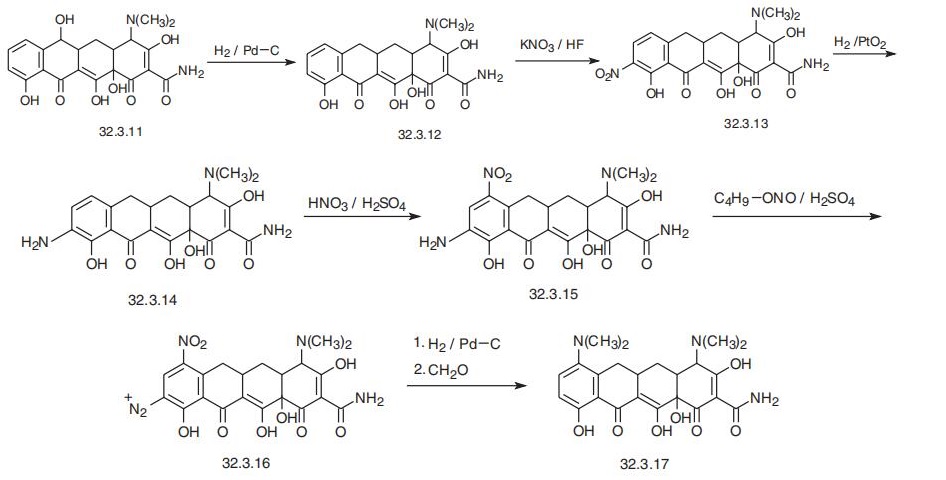
| | Drug interactions | Potentially hazardous interactions with other drugs
Anticoagulants: possibly enhanced anticoagulant
effect of coumarins and phenindione.
Oestrogens: possibly reduced contraceptive effect of
oestrogens (risk probably small)
.
Retinoids: possibly increased risk of benign
intracranial hypertension - avoid. | | Metabolism | Undergoes some metabolism in the liver, mainly to
9-hydroxyminocycline. | | Dosage forms | Up to 200 mg daily in divided doses. |
| | Minocycline Preparation Products And Raw materials |
|