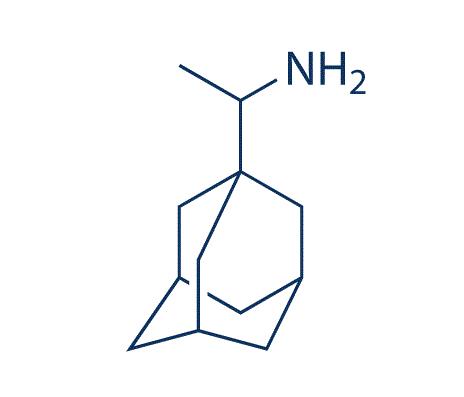
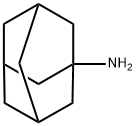
Mechanism and toxicity of Amantadine
Apr 11,2022
Amantadine (1-adamantanamine hydrochloride) is a tricyclic amine compound that was first synthesized from its precursor adamantane in 1941. The drug is active against type A influenza viruses, but not B or C types, and is useful for seasonal chemoprophylaxis, for outbreak control in institutionalized populations, and as a therapeutic agent against infection with all strains of influenza A virus. Amantadine was initially approved in the USA in 1966 for prophylaxis only against influenza A/H2N2; it was not until ten years later that it was approved for prophylactic and therapeutic use against all influenza A subtypes – perhaps the fatal blow to the drug’s commercial success.
Mechanism of action
The main mechanism by which adamantanes inhibit the replication of influenza A virus is by blocking hydrogen ion flow through the influenza M2 protein proton pore; the topic has recently been reviewed in detail. The M2 protein is a homo-tetrameric protein, one of three influenza envelope proteins [the others being the hemagglutinin (HA) and the neuraminidase (NA)] which is essential for efficient virus replication in cell culture and presumably in vivo as well.
Although the adamantane antivirals may inhibit replication of influenza and other viruses by interaction with other targets in the virus life cycle, these are not thought to be relevant at clinically achievable concentrations. Hence, understanding the mechanism of action of adamantanes requires an understanding of the influenza virus life cycle and the relevance of the M2 protein in it. The influenza A virus enters cells by first binding to them through an interaction between the virion HA protein and cell surface sialic (neuraminic) acid residues.
This triggers receptor-mediated endocytosis, delivering the virus particles to cellular endosomes. These processes are not altered by amantadine or rimantadine. Similarly, amantadine does not appear to act at this step in the replicative cycle of other viruses that are susceptible to the drug in higher concentrations. To initiate virion transcription and translation, the replicative complex of the virus (the polymerase and associated proteins) must move from the interior of the influenza virion in the endosome to the cytosol. For this to occur, the viral envelope must fuse with the membranes of the endosome in which it is contained through a low pH-induced conformational change in the viral HA. It is in this process that the M2 protein, which is a hydrogen ion channel, is critical.
Toxicity
The most commonly encountered side-effects of amantadine are CNS symptoms, such as nervousness, difficulty in concentration, confusion, insomnia, light-headedness, dizziness, tremor, slurred speech, ataxia, drowsiness, depression, hallucinations, and headache; these symptoms are reported by up to 11–33% of young adults who take amantadine at a dose of 100mg twice daily. In contrast, in some clinical trials using amantadine for prophylaxis or in volunteer studies, at a dose of 200mg daily, the frequency of side-effects has been in the order of 7–10%. Psychiatric syndromes rarely associated with amantadine include frank psychosis, mania, and a single case of pathologic jealousy (Othello syndrome) in a patient receiving amantadine that was ascribed to the drug. In addition, two cases of peripheral neuropathy attributed to amantadine have recently been reported.
Grand mal seizures are an unusual manifestation of amantadine toxicity, but may be seen occasionally at the usual dose employed for prophylaxis or perhaps more commonly at higher doses. Status epiliepticus was reported in a child with an amantadine overdose. As increases in seizures have also been reported in patients with a past history of seizures who take amantadine, such persons should be monitored closely during amantadine therapy.
- Related articles
- Related Qustion
- Amantadine: antiviral and anti-Parkinson's double guard Sep 5, 2024
This article will introduce basic information, pharmacological action, clinical application, side effects, and precautions of amantadine.
Hydroxylamine hydrochloride is an inorganic substance, a colorless crystalline, easily deliquescence, white chemical substance, mainly used as a reducing agent and an imaging agent....
Apr 11,2022APIRimantadine is an orally administered antiviral drug used to treat, and in rare cases prevent, influenzavirus A infection. When taken within one to two days of developing symptoms, rimantadine can shorten the duration and moderate the se....
Apr 11,2022APIAmantadine
768-94-5You may like
- Amantadine
-
- 2025-12-14
- CAS:768-94-5
- Min. Order:
- Purity: 0.99
- Supply Ability:
- Amantadine
-

- $0.00 / 25Kg/Drum
- 2025-12-13
- CAS:768-94-5
- Min. Order: 1KG
- Purity: 98%min
- Supply Ability: 1000kg
- Amantadine
-

- $5.00/ KG
- 2025-12-12
- CAS:768-94-5
- Min. Order: 1KG
- Purity: 99% hplc
- Supply Ability: 500TONS