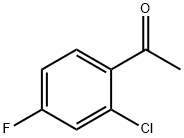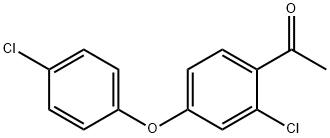
1-[2-Chloro-4-(4-chlorophenoxy)phenyl]ethan-1-one synthesis
- Product Name:1-[2-Chloro-4-(4-chlorophenoxy)phenyl]ethan-1-one
- CAS Number:119851-28-4
- Molecular formula:C14H10Cl2O2
- Molecular Weight:281.13
6842-62-2
172 suppliers
inquiry

75-36-5
589 suppliers
$17.92/100G
![1-[2-Chloro-4-(4-chlorophenoxy)phenyl]ethan-1-one](/CAS/GIF/119851-28-4.gif)
119851-28-4
273 suppliers
$8.00/5g
Yield:119851-28-4 94.7%
Reaction Conditions:
with Aluminum Chloride in 1,2-dichloro-ethane at -15; for 6 h;Temperature;Solvent;Reagent/catalyst;
Steps:
1.1-1.4; 2.1-2.4; 3.1-3.4; 4.1-4.4; 5.1-5.4; 6.1-6.4; 7.1-7.4; 8.1-8.4 Experimental example 4
(1) Put 75.0 g of diphenyl ether (98.5%) into a 1000 ml four-necked flask, 90.2 g of dichloroethane as a solvent and 49.2 g of aluminum trichloride as a catalyst were added, and the temperature was lowered to -15°C. 30.0g of acetyl chloride and 90.0g of dichloroethane were prepared into a homogeneous solution, Use a metering pump to pump into the reaction system, control the reaction dropwise addition time of 2h, and the holding time of 4h. After the heat preservation is over. A small amount of reaction solution is hydrolyzed to obtain an oil layer, Normalized gas spectrum detection, when the diphenyl ether content is less than 1%, Change the synthesis device to a distillation device, Remove 171g of dichloroethane (95% fraction) under negative pressure, The residue in the kettle is 134.5 g of black solid particulate matter. (2) Add 380g of deionized water to a 1000mL four-necked flask, 20g reagent hydrochloric acid, cooled to 5°C, Slowly put the above-mentioned 134.5g solid into the water, after the hydrolysis is completely finished, Incubate at 70°C for 0.5h, and stratify while hot. 91.8 g of an oil layer was obtained. Water layer 420.5g (3) Wash the oil layer in (2) with 50 g of deionized water at room temperature once to obtain 90 g of the oil layer after washing. The obtained washing water 51.4g was mixed with (2) After the hydrolysis, the water layers were combined and extracted once with 240 g of dichloroethane, After extraction, the oil layer is evaporated to recover the solvent, and then reused after drying. After evaporation, 3.3 g of the residue in the still obtained was combined with the oil layer after washing with water in (3). (4) 93.3g of combined oil layers in (3) were dissolved in 165g of petroleum ether, The temperature was raised to 70°C, and after 15min of heat preservation, the temperature was lowered to crystallize. Suction filtration at 10°C to obtain 82.68 g of purified diphenyl ether ketone solid. The result is normalized by gas spectrum, and the normalized result of the finished product is 99.5%. The overall yield was 94.7%.
References:
CN113816838,2021,A Location in patent:Paragraph 0005; 0025-0072
106-46-7
560 suppliers
$10.00/5g
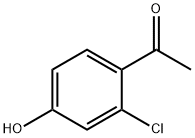
68301-59-7
111 suppliers
$45.00/1g
![1-[2-Chloro-4-(4-chlorophenoxy)phenyl]ethan-1-one](/CAS/GIF/119851-28-4.gif)
119851-28-4
273 suppliers
$8.00/5g
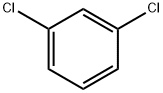
541-73-1
411 suppliers
$20.00/25G
![1-[2-Chloro-4-(4-chlorophenoxy)phenyl]ethan-1-one](/CAS/GIF/119851-28-4.gif)
119851-28-4
273 suppliers
$8.00/5g
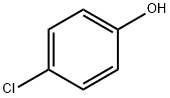
106-48-9
497 suppliers
$10.00/5G
![1-[2-Chloro-4-(4-chlorophenoxy)phenyl]ethan-1-one](/CAS/GIF/119851-28-4.gif)
119851-28-4
273 suppliers
$8.00/5g