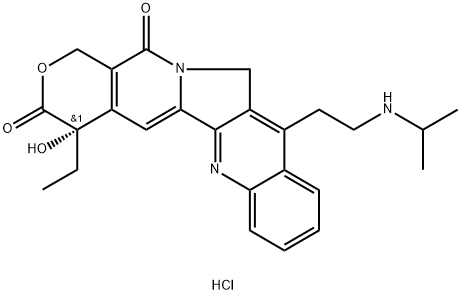
Camtobell hydrochloride synthesis
- Product Name:Camtobell hydrochloride
- CAS Number:213819-48-8
- Molecular formula:C25H28ClN3O4
- Molecular Weight:469.97
7689-03-4
597 suppliers
$18.00/1g

213819-48-8
108 suppliers
$23.00/1mg
Yield:-
Steps:
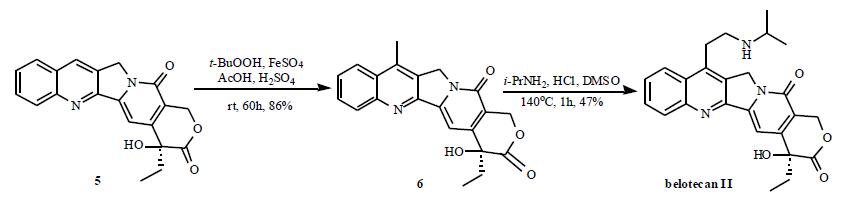
Multi-step reaction with 2 steps
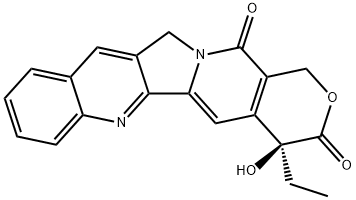
1: 86 percent / ferrous sulfate heptahydrate; tert-butylhydroperoxide; conc. H2SO4 / H2O / 60 h / 20 °C
2: 47 percent / conc. HCl / 1 h / 140 °C
References:
Ahn, Soon Kil;Choi, Nam Song;Jeong, Byeong Seon;Kim, Kye Kwang;Journ, Duck Jin;Kim, Joon Kyum;Lee, Sang Joon;Kim, Jung Woo;Hong, Chung Il;Jew, Sang-sup [Journal of Heterocyclic Chemistry,2000,vol. 37,# 5,p. 1141 - 1144]
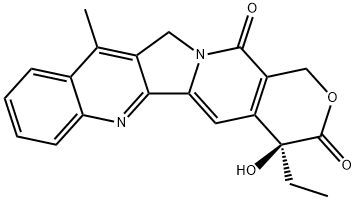
78287-26-0
28 suppliers
inquiry
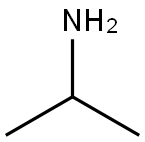
75-31-0
439 suppliers
$14.00/25mL

77-78-1
321 suppliers
$19.69/1ml

213819-48-8
108 suppliers
$23.00/1mg