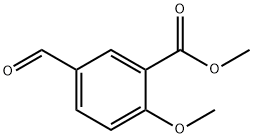
Methyl 5-formyl-2-methoxybenzoate synthesis
- Product Name:Methyl 5-formyl-2-methoxybenzoate
- CAS Number:78515-16-9
- Molecular formula:C10H10O4
- Molecular Weight:194.18
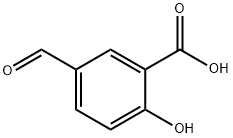
616-76-2

74-88-4

78515-16-9
General procedure for the synthesis of methyl 5-formyl-2-methoxybenzoate from 5-formyl-2-hydroxybenzoic acid and iodomethane: 5-formyl-2-hydroxybenzoic acid (2.0 g, 12.0 mmol), iodomethane (1.5 mL, 25 mmol) and potassium carbonate (3.06 g, 22.2 mmol) were mixed in dimethylformamide (15 mL). The reaction mixture was stirred at room temperature for 16 hours. After completion of the reaction, the solvent was removed by distillation under reduced pressure. The crude product was dissolved in ethyl acetate, washed sequentially with water (2×), saturated saline (2×) and the organic phase was dried with anhydrous sodium sulfate. After filtration, the filtrate was concentrated under reduced pressure and the resulting residue was purified by column chromatography to afford the target product methyl 5-formyl-2-methoxybenzoate 1.85 g (79% yield). The structure of the product was confirmed by 1H-NMR (CDCl3, 500 MHz) and mass spectrometry: 1H-NMR δ 9.91 (s, 1H), 8.31 (d, J = 2.1 Hz, 1H), 8.02 (dd, J = 8.5, 2.5 Hz, 1H), 7.11 (d, J = 8.6 Hz, 1H), 3.99 (s, 3H), 3.91 (s, 3H); mass spectrometry: 195 (s, 3H); mass spectrometry: 196 (s, 3H); mass spectrometry: 196 (s, 3H). 3H); MS: 195.05 (MH)+.
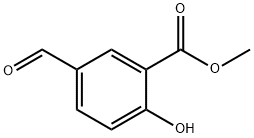
41489-76-3
139 suppliers
$8.00/1g

74-88-4
356 suppliers
$15.00/10g

78515-16-9
186 suppliers
$50.00/10g
Yield:78515-16-9 98%
Reaction Conditions:
Stage #1: methyl-5-formyl-2-hydroxybenzoatewith potassium carbonate in N,N-dimethyl-formamide at 80; for 0.5 h;
Stage #2: methyl iodide in N,N-dimethyl-formamide at 80; for 6 h;
Steps:
8.1 Step 1: Preparation of methyl 5-formyl-2-methoxybenzoate
Dissolve methyl 5-formyl-2-hydroxybenzoate (500.0 mg, 2.8 mmol) and potassium carbonate (1.2 g, 8.3 mmol) in DMF (10 mL), heat to 80 ° C for 0.5 hour, and then add methyl iodide (787.9 mg, 5.6 mmol) and maintained at 80 ° C for 6 hours. The reaction solution was cooled to room temperature, an appropriate amount of water was added, and extraction was performed 3 times with ethyl acetate. The organic phases were combined and washed with a saturated sodium chloride solution, dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated to give the title compound (530.0 mg, yield: 98%) in this step
References:
CN110724075,2020,A Location in patent:Paragraph 0283; 0284; 0285; 0286
75-75-2
651 suppliers
$10.00/5g
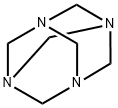
100-97-0
0 suppliers
$14.00/250G
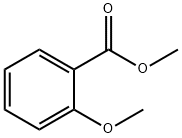
606-45-1
262 suppliers
$9.00/10g

78515-16-9
186 suppliers
$50.00/10g
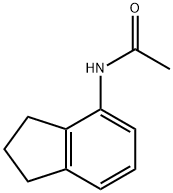
77802-37-0
3 suppliers
inquiry

78515-16-9
186 suppliers
$50.00/10g
