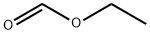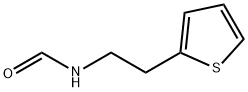
N-[2-(2-Thienyl)ethyl]forMaMide synthesis
- Product Name:N-[2-(2-Thienyl)ethyl]forMaMide
- CAS Number:28783-49-5
- Molecular formula:C7H9NOS
- Molecular Weight:155.22
Yield:28783-49-5 97%
Reaction Conditions:
with sodium hydrogencarbonate at 20;
Steps:
N-(2-(thiophen-2-yl)ethyl)formamide
The title compound was prepared according toGP1-1. To 2-thiophene ethylamine (2.5 g, 20 mmol) were added ethylformate (10 mL) and NaHCO3 (1 g). The mixture was stirred at rt for overnight.Water was added, extracted by EA (2x25 mL), dried over Na2SO4,and concentrated under reduced pressure to get brown oil (3.0 g, 97% yield).LC-MS (ESI): [M+1]+= 156.20, tR = 0.43 min.1H NMR(400 MHz, CDCl3) δ minor isomer was observed and only the major wasrecorded herein, 8.07 (s, 1H), 7.14 (d, J= 5.1 Hz, 1H), 6.92 (dd, J = 4.9, 3.6Hz, 1H), 6.83 (d, J = 2.5 Hz, 1H),6.49 (brs, 1H), 3.53 (q, J = 6.6 Hz,2H), 3.03 (t, J = 6.9 Hz, 2H).13C NMR(100 MHz, CDCl3) δ minor isomer was observed and only the major wasrecorded herein, 161.5, 141.0, 127.1, 125.4, 124.0, 39.5, 29.8.
References:
Lv, Fengping;Li, Zhi-Fang;Hu, Wenhao;Wu, Xiaohua [Bioorganic and Medicinal Chemistry,2015,vol. 23,# 24,p. 7661 - 7670] Location in patent:supporting information
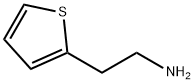
30433-91-1
360 suppliers
$5.00/5g

64-18-6
1131 suppliers
$22.12/250ML
![N-[2-(2-Thienyl)ethyl]forMaMide](/CAS/20150408/GIF/28783-49-5.gif)
28783-49-5
8 suppliers
inquiry
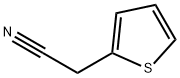
20893-30-5
196 suppliers
$9.00/1g
![N-[2-(2-Thienyl)ethyl]forMaMide](/CAS/20150408/GIF/28783-49-5.gif)
28783-49-5
8 suppliers
inquiry
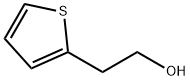
5402-55-1
506 suppliers
$5.00/5g
![N-[2-(2-Thienyl)ethyl]forMaMide](/CAS/20150408/GIF/28783-49-5.gif)
28783-49-5
8 suppliers
inquiry