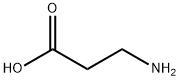
β-Alanine
- CAS No.
- 107-95-9
- Chemical Name:
- β-Alanine
- Synonyms
- BETA-ALANINE;3-AMINOPROPANOIC ACID;β-Ala;B-ALANINE;H-β-Ala-OH;3-AMINOPROPIONIC ACID;BETA-ALA;Analine;3-aminopropanoic;Beta-alaine
- CBNumber:
- CB0711205
- Molecular Formula:
- C3H7NO2
- Molecular Weight:
- 89.09
- MDL Number:
- MFCD00008200
- MOL File:
- 107-95-9.mol
- MSDS File:
- SDS
| Melting point | 202 °C (dec.)(lit.) |
|---|---|
| Boiling point | 237.1±23.0 °C(Predicted) |
| Density | 1,437 g/cm3 |
| refractive index | 1.4650 (estimate) |
| FEMA | 3252 | BETA-ALANINE |
| Flash point | 204-206°C |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | H2O: 1 M at 20 °C, clear, colorless |
| pka | 3.55(at 25℃) |
| form | Crystalline Powder |
| color | White |
| PH | 6.0-7.5 |
| Odor | at 100.00 %. odorless |
| Odor Type | odorless |
| Water Solubility | Soluble in water(550g/L). Slightly soluble in alcohol. Insoluble in ether and acetone. |
| Decomposition | 204-206 ºC |
| λmax |
λ: 260 nm Amax: ≤0.02 λ: 280 nm Amax: ≤0.02 |
| JECFA Number | 1418 |
| Merck | 14,205 |
| BRN | 906793 |
| Stability | Stable. Keep dry. |
| InChIKey | UCMIRNVEIXFBKS-UHFFFAOYSA-N |
| LogP | -0.882 (est) |
| Substances Added to Food (formerly EAFUS) | BETA-ALANINE |
| CAS DataBase Reference | 107-95-9(CAS DataBase Reference) |
| FDA UNII | 11P2JDE17B |
| NIST Chemistry Reference | Beta-alanine(107-95-9) |
| EPA Substance Registry System | .beta.-Alanine (107-95-9) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P261-P305+P351+P338 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36/37/38 | |||||||||
| Safety Statements | 24/25-36-26 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | UA2369200 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 29224920 | |||||||||
| NFPA 704 |
|
β-Alanine price More Price(61)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 05159 | β-Alanine BioUltra, ≥99.0% (NT) | 107-95-9 | 100g | $78 | 2024-03-01 | Buy |
| Sigma-Aldrich | 05159 | β-Alanine BioUltra, ≥99.0% (NT) | 107-95-9 | 500g | $275 | 2023-06-20 | Buy |
| TCI Chemical | A0180 | Beta Alanine >99.0%(T) | 107-95-9 | 25g | $21 | 2024-03-01 | Buy |
| TCI Chemical | A0180 | Beta Alanine >99.0%(T) | 107-95-9 | 500g | $60 | 2024-03-01 | Buy |
| Alfa Aesar | A16665 | beta-Alanine, 98% | 107-95-9 | 250g | $29.9 | 2024-03-01 | Buy |
β-Alanine Chemical Properties,Uses,Production
Description
Beta-alanine is a non-proteogenic amino acid that is produced endogenously in the liver. In addition, humans acquire beta-alanine through the consumption of foods such as poultry and meat. By itself, the ergogenic properties of beta-alanine are limited; however, beta-alanine has been identified as the rate-limiting precursor to carnosine synthesis, and has been consistently shown to increase levels of carnosine in human skeletal muscle. Doses of 4 to 6 g/day of beta-alanine have been shown to increase muscle carnosine concentrations by up to 64 % after 4 weeks, and up to 80 % after 10 weeks. Baguet et al. demonstrated that individuals vary in the magnitude of response to 5 to 6 weeks of betaalanine supplementation (4.8 g/day), with high responders increasing muscle carnosine concentrations by an average of 55 %, and low responders increasing by an average of only 15 %. The difference between high and low responders seems, at least in part, to be related to baseline muscle carnosine content and muscle fiber composition.

β-Alanine, a β−amino acid, is a component of pantothenic acid and the rate-limiting amino acid in the biosynthesis of the histidinyl antioxidant dipeptides carnosine and anserine. Endogenous β-amino acid that is a nonselective agonist at glycine receptors and a ligand for the G protein-coupled orphan receptor, TGR7 (MrgD). β-Alanine flux plays a cytoprotective role by supporting the osmotic stability of marine organisms, preimplantation mouse embryos and mammalian cells exposed to hypoxic stress.
Chemical Properties
White powder
Uses
It is widely used in medicine, feed, food, and other industries, mostly to synthesize pantothenic acid and calcium pantothenate (a medicine and feed additive), carnosine, pamidronate sodium, barley nitrogen. It is also used to produce plating corrosion inhibiter, as a biological reagent, and as an organic synthesis intermediate. Used as a food and health supplement additive. Endogenous beta-amino acids, non-selective glycine receptor agonists ,G-protein-coupled orphan receptor (TGR7, MrgD) ligand. Relying on the stability of marine biology, beta-aminopropionic acid has a protective effect on cells.
Biological Functions
β-alanine is a non-essential amino acid that can potentially indirectly enhance performance of extremely high intensity (110% of VO2 peak), short duration (1-5 minutes) bouts of exercise. β-alanine may enhance performance by increasing intramuscular levels of another amino acid, carnosine. It is well established that acidosis can increase fatigue during exercise and therefore increasing the body’s buffering capacity may improve high-intensity, short-duration exercise performance. The importance of carnosine has been previously described by Tallon et al., who reported carnosine concentrations in body builders of 40 mmol/kg dry mass compared to the average human of 16 mmol/kg dry mass. Tallon et al. estimated carnosine to account for 20% of total buffering capacity in body builders compared to 10% in the typical population. In theory, if an athlete of any age increases the amount of carnosine present in skeletal muscle, they can enhance their ability to buffer acidic concentrations during high-intensity exercise and thus delay fatigue.
Preparation
- Acrylonitrile and ammonia react in a solution of diphenylamine and t-butanol to create beta-aminopropionitrile, which is then alkalized to obtain beta-aminopropionic acid. In a dry autoclave, sequentially add acrylonitrile, diphenylamine and t- butanol, and stir for 5min. Then, add liquid ammonia, maintain the temperature at 100-109℃ and pressure at 1MPa, and stir for 4h. Cool to below 10℃ and stop mixing when the pressure reaches atmospheric pressure. At 65-70℃/(8.0-14.7kPa), decrease the pressure to recover t-butanol to obtain crude beta-aminopropionitrile. Distill the crude product under low pressure, collect the 66-105℃/(1.33-4.0kPa) distillation to obtain beta-aminopropionitrile, and maintain temperature for 1h. Steam under low pressure for half an hour to remove the ammonia in the reaction solution, add water, and drop in hydrochloric until PH reaches 7-7.2. Filter to remove trace impurities. Concentrate the filtered liquid until a large amount of solid precipitates, extract while hot and cool to below 10℃. Filter and vacuum dry to obtain beta-aminopropionic acid. This method requires 982kg beta-aminopropionitrile for every ton of product, and the yield of alkalization is 90%.
- Place the alkaline sodium hypochlorite solution obtained from degradation of succinimide (Hoc reaction) (containing 14% sodium hypochlorite, 8% sodium hydroxide, 30% sodium carbonate) and ice into a reaction chamber, mix and add succinimide, and let react at 18-25℃ for 0.5h. Increase temperature to 40-50℃ and allow to react for 1h. Add hydrochloric acid to adjust the PH to 4-5, decrease the pressure to condense. After condensed and cooled, add 3 times the amount of 95% ethanol to allow inorganic salts to precipitate, filter, and repeat once again. Then, dilute the filtered liquid with 4 times the amount of distilled water and reflux for 1h. Add activated charcoal to remove color, filter, and pass the filtered liquid through exchange resin. Add activated charcoal to remove color, filter, decrease pressure to condense, cool to crystalize, filter, use distilled water to recrystallize once, and obtain beta-aminopropionic acid.
- Hydrolyze and acidify beta-aminopropionitrile to obtain.
beta-alanine dosage
A common side effect of β-alanine supplementation is paresthesia which is a sensation of tingling, burning, prickling or numbness to a person's skin with no apparent long-term physical effects.
An early investigation reported 10 mg/kg or approximately 800 mg of β-alanine to be the maximal single dose that could be consumed without experiencing significant symptoms of paresthesia. Moreover, β-alanine concentrations peaked 30–40 minutes following consumption and returned to baseline 3 hours following consumption. As a result, an individual supplementing with β-alanine could consume multiple doses throughout the day in 3-hour intervals. In this regard, the consumption of 6.4 g/day of β-alanine consumed in eight doses of 800 mg appears more effective at enhancing skeletal muscle carnosine levels than the consumption of 3.2 g/day of β-alanine consumed in four doses of 800 mg. Finally, controlled release capsules have been developed allowing for the single dose consumption of 1600 mg without the presentation of paresthesia, allowing users to consume fewer daily doses of β-alanine. As a result, masters athletes wishing to experiment with β-alanine should aim to consume 6.4 g/day, which can be accomplished with eight doses of 800 mg of β-alanine, or with four doses of 1600 mg with controlled release β-alanine capsules.
Description
β-Alanine (or beta-alanine) is a naturally occurring beta amino acid, which is an amino acid in which the amino group is at the β- position from the carboxylate group (i.e., two atoms away. The IUPAC name for β-alanine is 3-amino propanoic acid. Unlike its counterpart α-alanine, β-alanine has no stereocenter.
β-Alanine is not used in the biosynthesis of any major proteins or enzymes. It is formed in vivo by the degradation of dihydrouracil and carnosine. It is a component of the naturally occurring peptides carnosine and anserine and also of pantothenic acid (vitamin B5), which itself is a component of coenzyme A. Under normal conditions, β-alanine is metabolized into acetic acid.
β-Alanine is the rate-limiting precursor of carnosine, which is to say carnosine levels are limited by the amount of available β-alanine. Supplementation with β-alanine has been shown to increase the concentration of carnosine in muscles, decrease fatigue in athletes and increase total muscular work done.
Typically, studies have used supplementing strategies of multiple doses of 400 mg or 800 mg, administered at regular intervals for up to eight hours, over periods ranging from 4 to 10 weeks . After a 10 - week supplementing strategy, the reported increase in intramuscular carnosine content was an average of 80.1% (range 18 to 205%).
Chemical Properties
This is a secondary amino acid, which is formed in vivo by the degradation of dihydrouracil and carnosine. Because neuronal uptake and neuronal receptor sensitivity to β-alanine have been demonstrated, the compound may be a false transmitter replacing GABA. A rare genetic disorder, hyper-β-alaninemia, has been reported. It is used as a flavor enhancer, flavoring agent, nutrient supplement or adjuvant. β-Alanine has a slightly sweet taste.
Chemical Properties
White crystalline powder
Occurrence
Reported to occur as a component in amino acids; carnosine, anserine, pantothenic acid
Uses
Effective catalyst for the Knoevenagel condensation. Beta Alanine is used as nutrition supplements in food production. Increase muscular strength & power output, Increases Muscle Mass, increase Anaerobic Endurance.
Uses
β-Alanine is a naturally occurring beta amino acid. β-Alanine is formed in vivo by the degradation of dihydrouracil (D449990) and carnosine. β-Alanine is also the rate-limiting precursor of carnosine, as a result supplementation with β-alanine increases the concentration of carnosine in muscles.
Uses
β-Alanine has been used as a ligand for the orphan MAS-related receptor. It has also been used in culture media used for certain strains of yeast to test for β-alanine auxotrophy.
Preparation
By heating acrylic acid with concentrated aqueous ammonia under pressure, by addition of acrylonitrile to phthalimide or to ammonia; from β-aminopropionitrile, from succinimide by the Hofmann degradation.
Definition
ChEBI: A naturally-occurring beta-amino acid comprising propionic acid with the amino group in the 3-position.
General Description
An N-blocked form of alanine.
Flammability and Explosibility
Non flammable
Biochem/physiol Actions
β-Alanine, a β?amino acid, is a component of pantothenic acid and the rate-limiting amino acid in the biosynthesis of the histidinyl antioxidant dipeptides carnosine and anserine. Endogenous β-amino acid that is a nonselective agonist at glycine receptors and a ligand for the G protein-coupled orphan receptor, TGR7 (MrgD). β-Alanine flux plays a cytoprotective role by supporting the osmotic stability of marine organisms, preimplantation mouse embryos and mammalian cells exposed to hypoxic stress.
Purification Methods
Crystallise β-alanine by dissolving it in a hot saturated aqueous solution, filtering, adding four volumes of absolute EtOH and cooling in an ice-bath. Recrystallise it in the same way and then finally, crystallise it from a warm saturated solution in 50% EtOH and adding four volumes of absolute EtOH with cooling in an ice-bath. The crystals are dried in a vacuum desiccator over P2O5. [Donovan & Kegeles J Am Chem Soc 83 255 1961, Beilstein 4 IV 2526.]
β-Alanine Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
1of4
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 | info@gihichemicals.com | China | 49999 | 58 |
| BLiT (Hefei)Chemical Co.,Ltd | +86-551-62622640 | sales@blitchem.com | China | 291 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5993 | 58 |
| Hebei Yime New Material Technology Co., Ltd. | +86-66697723 +86-17703311139 | admin@china-yime.com | China | 563 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | deasea125996@gmail.com | China | 2503 | 58 |
| Zibo Hangyu Biotechnology Development Co., Ltd | +86-0533-2185556 +8617865335152 | Mandy@hangyubiotech.com | China | 11013 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 7845 | 58 |
| airuikechemical co., ltd. | +undefined86-15315557071 | sales02@airuikechemical.com | China | 994 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +8618740459177 | sarah@tnjone.com | China | 893 | 58 |
| Ouhuang Engineering Materials (Hubei) Co., Ltd | +8617702722807 | admin@hbouhuang.com | China | 2259 | 58 |
View Lastest Price from β-Alanine manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-26 | beta-Alanine
107-95-9
|
US $0.00 / KG | 1KG | 99% | 1000mt/year | Jinan Finer Chemical Co., Ltd | |
 |
2024-04-26 | β-Alanine
107-95-9
|
US $10.00 / kg | 1kg | 99 | 10000kg/month | Hebei Yime New Material Technology Co., Ltd. | |
 |
2024-04-26 | β-Alanine
107-95-9
|
US $10.00 / kg | 1kg | 99 | 1000kg/week | Hebei Yime New Material Technology Co., Ltd. |
-

- beta-Alanine
107-95-9
- US $0.00 / KG
- 99%
- Jinan Finer Chemical Co., Ltd
-

- β-Alanine
107-95-9
- US $10.00 / kg
- 99
- Hebei Yime New Material Technology Co., Ltd.
-

- β-Alanine
107-95-9
- US $10.00 / kg
- 99
- Hebei Yime New Material Technology Co., Ltd.