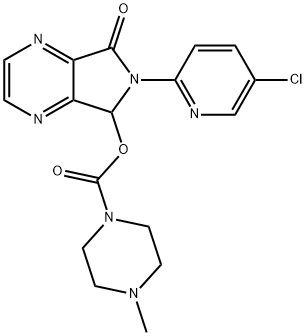
Zopiclone
- CAS No.
- 43200-80-2
- Chemical Name:
- Zopiclone
- Synonyms
- imovane;Zopiclone R-IsoMer CIV;6-(5-Chloro-2-pyridinyl)-7-oxo-6,7-dihydro-5H-pyrrolo[3,4-b]pyrazin-5-yl 4-methyl-1-piperazinecarboxylate;Imobam;Amoban;Amovane;Sopivan;Limovan;Ximovan;CS-1331
- CBNumber:
- CB1401303
- Molecular Formula:
- C17H17ClN6O3
- Molecular Weight:
- 388.81
- MDL Number:
- MFCD00133931
- MOL File:
- 43200-80-2.mol
| Melting point | 1780C |
|---|---|
| Boiling point | 580.7±50.0 °C(Predicted) |
| Density | 1.1105 (estimate) |
| Flash point | 2℃ |
| storage temp. | Store at RT |
| solubility | DMSO: 2 mg/mL |
| form | Solid |
| pka | pKa ﹣1.5±0.1(10% ACN in aq. H2SO4 t = 25.0) (Uncertain) |
| color | White to Off-White |
| Stability | Hygroscopic |
| InChIKey | GBBSUAFBMRNDJC-UHFFFAOYSA-N |
| CAS DataBase Reference | 43200-80-2(CAS DataBase Reference) |
| FDA UNII | 03A5ORL08Q |
| NCI Drug Dictionary | Amoban |
| ATC code | N05CF01 |
| NIST Chemistry Reference | Zopiclone(43200-80-2) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302-H336 | |||||||||
| Precautionary statements | P261-P264-P270-P271-P301+P312-P304+P340+P312 | |||||||||
| Hazard Codes | Xn,Xi,F | |||||||||
| Risk Statements | 20/21/22-36/37/38-62-36-11 | |||||||||
| Safety Statements | 26-36-36/37-16 | |||||||||
| RIDADR | UN 1648 3 / PGII | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | TL1425000 | |||||||||
| HS Code | 29339900 | |||||||||
| Toxicity | mouse,LD50,intramuscular,541mg/kg (541mg/kg),LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSIONBEHAVIORAL: CHANGES IN MOTOR ACTIVITY (SPECIFIC ASSAY),Oyo Yakuri. Pharmacometrics. Vol. 26, Pg. 935, 1983. | |||||||||
| NFPA 704 |
|
Zopiclone Chemical Properties,Uses,Production
Description
Zopiclone is an agonist at the type A γ-aminobutyric acid (GABA) receptor. It is a non-benzodiazepine hypnotic which was first reviewed in Drugs in 1986 and it is indicated for short-term treatment of insomnia. Zopiclone has a relatively low propensity to cause residual clinical effects (such as difficulty in waking or reduced morning concentration).
References
[1] Stuart Noble, Heather D. Langtry and Harriet M. Lamb, Drugs, 1998, vol. 55, 277-302
[2] G. Hajak, W. E. Müller, H. U. Wittchen, D. Pittrow, W. Kirch, Abuse and dependence potential for the non-benzodiazepine hypnotics zolpidem and zopiclone: a review of case reports and epidemiological data, Addiction, 2003, vol. 98, 1371-1378
Description
Zopiclone is an effective hypnotic agent with a short duration of action. Although it interacts with the benzodiazepine receptor complex, it is reported to have minimal effects on memory, little synergy with alcohol, and low abuse potential.
Chemical Properties
Crystalline Solid
Originator
Rhone-Poulenc (France)
Uses
antihyperammonemic,antineoplastic
Uses
inhibits tyrosinase and prevents melanin formation used to whiten and lighten skin
Uses
Cyclopyrrolone member of a family of non-benzodiazepine GABAA receptor agonists. This is a controlled substance (depressant) in the US but not in Canada. Sedative, hypnotic
Definition
ChEBI: A pyrrolo[3,4-b]pyrazine compound having a 4-methylpiperazine-1-carboxyl group at the 5-position, a 5-chloropyridin-2-yl group at the 6-position and an oxo-substituent at the 7-position.
Manufacturing Process
Producing of 6-(5-chloropyrid-2-yl)-5-(4-methylpiperazin-1-yl)-carbonyloxy-7-
oxo-5,6-dihydropyrrolo[3,4-b]pyrazine by two methods.
1). A solution of 6-(5-chloropyrid-2-yl)-5-hydroxy-7-oxo-5,6-
dihydropyrrolo[3,4-b]pyrazine (12.0 g) in anhydrous dimethylformamide (360
ml) is added to a suspension of sodium hydride (50% dispersion in mineral
oil) (2.4 g) in anhydrous dimethylformamide (60 ml), whilst maintaining the
temperature at about -10°C. When the evolution of gas has ceased, a solution
of 1-chlorocarbonyl-4-methylpiperazine (8.1 g) in anhydrous
dimethylformamide (20 ml) is added, whilst maintaining the temperature at
about -10°C. The reaction mixture is stirred for a further 3 h whilst allowing it
to heat up gradually to a temperature of about 20°C, and then it is poured
into ice-water (1540 ml). The product which crystallizes is filtered off, washed
with water (150 ml) and then with diisopropyl ether (100 ml). After drying, a
product is obtained and is dissolved in ethyl acetate (600 ml). The solution
obtained is filtered through silica gel (250.0 g). Elution is then carried out with
ethyl acetate (3200 ml) followed by a mixture of ethyl acetate and methanol
The eluates are combined and concentrated to dryness under reduced
pressure. So 8.3 g of the 6-(5-chloropyrid-2-yl)-5-(4-methylpiperazin-1-yl)-
carbonyloxy-7-oxo-5,6-dihydropyrrolo[3,4-b]pyrazine are obtained, melting
point 178°C (recrystallisation from a mixture of acetonitrile and diisopropyl
ether 1:1; 190 ml).
2). 1-Methylpiperazine (155.0 g) is added to a suspension of 6-(5-chloropyrid-
2-yl)-7-oxo-5-phenoxycarbonyloxy-5,6-dihydropyrrolo[3,4-b]pyrazine (194.0
g) in acetone (970 ml) cooled to a temperature of about 3°C. The reaction
mixture is stirred for 3 h at a temperature of about 3°C and is then poured
into water (5000 ml). The product which precipitates is filtered off and then
washed with water (600 ml) and dried. This product is treated with methylene
chloride (1100 ml) at a temperature of about 20°C. The insoluble material is
filtered off and then the filtrate is washed with 1 N sodium hydroxide solution
(3x200 ml) and with water (3x200 ml). The organic phase is treated with
decolorizing charcoal (10.0 g), dried over potassium carbonate, filtered and
then concentrated to dryness under reduced pressure. The oily residue
obtained is dissolved in boiling acetonitrile (500 ml). The 101.0 g of 6-(5-
chloropyrid-2-yl)-5-(4-methylpiperazin-l-yl)carbonyloxy-7-oxo-5,6-
dihydropyrrolo[3,4-b]-pyrazine are obtained, melting point 178°C (washed
with ice cold acetonitrile, 50 ml, and then crystallizes with diisopropyl ether,
50 ml).
brand name
IMOVANE
Therapeutic Function
Sedative, Hypnotic
World Health Organization (WHO)
Zopiclone was introduced as a sedative in 1985. It remains registered in several countries and the World Health Organization is not aware of any other country that has refused registration.
Clinical Use
Hypnotic
Drug interactions
Potentially hazardous interactions with other drugs
Antibacterials: metabolism inhibited by
erythromycin; concentration significantly reduced by
rifampicin.
Antipsychotics: enhanced sedative effects.
Antivirals: concentration possibly increased by
ritonavir.
Metabolism
Zopiclone is extensively metabolised in the liver via the cytochrome P450 isoenzyme CYP3A4 and, to a lesser extent, CYP2C8; the 2 major metabolites, the less active zopiclone N-oxide and the inactive N-desmethylzopiclone, are excreted mainly in the urine. About 50
% of a dose is converted by decarboxylation to inactive metabolites, which are partly eliminated via the lungs as carbon dioxide.
Zopiclone Preparation Products And Raw materials
Raw materials
1of4