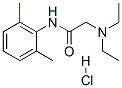
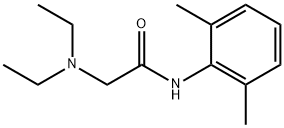
Lidocaine hydrochloride
- CAS No.
- 73-78-9
- Chemical Name:
- Lidocaine hydrochloride
- Synonyms
- LIDOCAINE HCL;LIGNOCAINE HYDROCHLORIDE;Lidocaine HCI;rucainahydrochloride;duncainehydrochloride;gravocainhydrochloride;leostesinhydrochloride;xylocitinhydrochloride;2-diethylamino-2’,6’-acetoxylididehydrochloride;alpha-diethylamino-2,6-acetoxylidinehydrochloride
- CBNumber:
- CB4117973
- Molecular Formula:
- C14H23ClN2O
- Molecular Weight:
- 270.8
- MDL Number:
- MFCD00034865
- MOL File:
- 73-78-9.mol
- MSDS File:
- SDS
| Melting point | 80-82°C |
|---|---|
| storage temp. | Inert atmosphere,2-8°C |
| Water Solubility | Water : ≥ 36 mg/mL (132.94 mM) |
| InChI | InChI=1S/C14H22N2O.ClH/c1-5-16(6-2)10-13(17)15-14-11(3)8-7-9-12(14)4;/h7-9H,5-6,10H2,1-4H3,(H,15,17);1H |
| InChIKey | IYBQHJMYDGVZRY-UHFFFAOYSA-N |
| SMILES | C(NC1=C(C)C=CC=C1C)(=O)CN(CC)CC.[H]Cl |
| LogP | 2.359 (est) |
| FDA 21 CFR | 310.545 |
| CAS DataBase Reference | 73-78-9(CAS DataBase Reference) |
| EWG's Food Scores | 1-3 |
| FDA UNII | EC2CNF7XFP |
| NCI Drug Dictionary | lidocaine hydrochloride |
| EPA Substance Registry System | Acetamide, 2-(diethylamino)-N-(2,6-dimethylphenyl)-, monohydrochloride (73-78-9) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302 |
| Precautionary statements | P264-P270-P301+P312-P330-P501 |
| RIDADR | 3249 |
| HazardClass | 6.1(b) |
| PackingGroup | III |
| Toxicity | LD50 oral in mouse: 220mg/kg |
Lidocaine hydrochloride price More Price(13)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| TRC | L397808 | Lidocaine hydrochloride | 73-78-9 | 5g | $60 | 2021-12-16 | Buy |
| TRC | L397808 | Lidocaine hydrochloride | 73-78-9 | 10g | $80 | 2021-12-16 | Buy |
| TRC | L397808 | Lidocaine hydrochloride | 73-78-9 | 1g | $40 | 2021-12-16 | Buy |
| ChemScene | CS-3888 | Lidocaine hydrochloride 99.46% | 73-78-9 | 500mg | $50 | 2021-12-16 | Buy |
| ChemScene | CS-3888 | Lidocaine hydrochloride 99.46% | 73-78-9 | 5g | $60 | 2021-12-16 | Buy |
Lidocaine hydrochloride Chemical Properties,Uses,Production
General
Lidocaine hydrochloride was synthesized by Löfgren and Lundquist in 1943, and was clinically introduced in 1948. It remains one of the most widely used local anaesthetics. It can be administered parenterally for a peripheral nerve block (PNB), intravenously, or applied topically at strengths of 2–4%. The addition of epinephrine 1:200 000 to 1:100 000 slows the vascular absorption of lidocaine and prolongs its effects.
Physical and chemical properties
Lidocaine hydrochloride is white and odorless crystal with bitter and numb taste. It is easily soluble in water, ethanol and organic solvents, but insoluble in ether. Aqueous solution in the case of acid and alkali do not break down, repeated autoclave rarely go bad.
Local anesthetic and antiarrhythmic drugs
Lidocaine hydrochloride is a local anesthetic and antiarrhythmic drug. It is clinically used for infiltration anesthesia, epidural anesthesia, surface anesthesia (including in the thoracoscopy or abdominal surgery for mucosal anesthesia) and nerve conduction block. The drug can also be used for acute myocardial infarction after ventricular premature beats and ventricular tachycardia, and for digitalis poisoning, cardiac surgery and ventricular arrhythmias caused by cardiac catheterization. But it is usually ineffective for supraventricular arrhythmias.
Lidocaine hydrochloride is an amide local anesthetic. After blood absorption or intravenous administration, the drug has obvious excitement and inhibition of biphasic effects for the central nervous system, and no excitement of the pioneer. With the dose increased, the role or toxicity increased, there is an anti-convulsive effect with sub-poisoning plasma concentration; Blood concentration of more than 5μg • ml-1 can occur convulsions. Lidocaine hydrochloride in low doses can promote outflow of K+ in cardiomyocytes, reduce myocardial autonomy, and has antiarrhythmic effects. In the treatment dose, lidocaine hydrochloride has no significant effect for the electrical activity of cardiomyocytes, atrioventricular conduction and myocardial contraction. Increased plasma concentration may cause slowing of heart conduction, atrioventricular block, inhibition of myocardial contractility and decreased cardiac output.
Application
Lidocaine hydrochloride is characterized by strong penetration, strong dispersion, rapidly onset. The anesthetic performance is twice that of procaine and the toxicity is1. There is an anesthetic effect after 5 minutes treatments, and anesthesia can last 1 to 1.5 hours, 50% longer than procaine. The drug is effective on the heart of the disease or arrhythmia caused by cardiac glycoside, but on the supraventricular tachycardia is poor. This product is fast and oral ineffective, with short duration, and often used as intravenous administration.
Metabolism
Lidocaine is metabolized by the liver with only a small amount (3%) found unchanged in urine. The three main mechanisms of metabolism are shown below: N-de-ethylation > monoethylglycinexylidide (MEGX) > glycinexylidide Hydrolysis of glycinexylidide 5-hydroxylation of lidocaine’s benzene ring Lidocaine possesses convulsant activity. Hepatic disease or reduced hepatic blood flow (as in congestive cardiac failure) will lower metabolic capacity.
Indications
- The drug can be used for infiltration anesthesia, epidural anesthesia, surface anesthesia and nerve conduction block
- The drug can be used for acute myocardial infarction after ventricular premature beats and ventricular tachycardia, and for digitalis poisoning, cardiac surgery and ventricular arrhythmias caused by cardiac catheterization. But it is usually ineffective for supraventricular arrhythmias.
Usage and dosage
Surface anesthesia with solution of 2% to 5%.Infiltration anesthesia with solution of 0.25% to 0.5%, conduction anesthesia with 2%, each injection point, horse, cattle 8 to 12 ml, sheep 3 to 4 ml. Epidural anesthesia with 2% solution, horse, cow, 8 to 12 ml, dog, cat, 0.22 ml per kilogram of body weight. Subcutaneous injection with 2% solution, pig, sheep, 80 ml, horse, cow, 400 ml, dog,25 ml, cat, 8.5 ml.
Treatment of arrhythmia, intravenous injection: Per kg of dog’s body weight of the initial dose is 2 to 4 mg, followed by 25 to 75 micrograms per minute intravenous infusion; Cat initial dose of 250 to 500 micrograms, followed by intravenous infusion of 20 micrograms per minute.
Adverse effect
The incidence of adverse effect with lidocaine hydrochloride was about 6.3%. Most adverse effects are dose dependent. Adverse effects are drowsiness, dizziness, nausea, vomiting, burnout, euphoria, insanity, muscle convulsions, syncope, blurred vision, confusion and difficulty breathing. Large doses lead to severe sinus bradycardia, cardiac arrest, severe atrioventricular block and weakened myocardial contractility, reduced blood pressure and so on. Excess concentrations of lidocaine hydrochloride in the blood cause some problems. For example, atrial conduction slows, atrioventricular blocks (A-V-B), and inhibits myocardial contractility and cardiac output decreases. There are little allergic effects, such as erythema rash, angioneurotic edema and so on.
Additional information
Lidocaine is contraindicated in patients with known hypersensitivity to local anaesthetics of the amide type and in patients with porphyria. Reactions due to overdose with lidocaine (high plasma levels) are systemic and involve the central nervous and cardiovascular systems. Effects include medullary depression, tonic and clonic convulsions, and cardiovascular collapse Solutions in multidose vials may contain hydrobenzoate derivatives and have been associated with allergic reactions in some patients. As with all of the amide local anaesthetics protein binding is reduced in the neonate (50% versus 64% in the adult), which necessitates reduced doses if adverse reactions are to be avoided.
Medicine interactions
- Cimetidine and β-blockers can inhibit metabolism of lidocaine through liver, so that the blood concentration increases and adverse reactions occur in the heart and nervous system. We should adjust the dose of lidocaine hydrochloride.
- Barbiturates can promote the metabolism of lidocaine hydrochloride, and the two drugs can cause bradycardia and sinus arrest.
- Combined with procainamide, the drug can produce excessive delirium and hallucinations, but does not affect the product plasma concentration.
- Isoprenaline Isoprinosine could increase the total clearance of lidocaine hydrochloride through increased liver blood flow; norepinephrine could reduce the total clearance of lidocaine hydrochlor through reduced liver blood flow.
- The drug is contraindicated with phenobarbital, thiopental sodium, sodium nitroprusside, mannitol, amphotericin B, ampicillin, and sulfadiazine.
Precautions
- Patients Allergic to other local anesthetics may be allergic to lidocaine hydrochloride.
- The following circumstances with caution: Pregnancy, neonatal especially in premature infants, liver blood flow reduction, liver and kidney dysfunction, congestive heart failure, severe myocardial damage, low blood volume, shock and other patients.
- Strictly grasp the concentration and total medication, excessive can cause convulsions and cardiac arrest; the body metabolism is slower than procaine, and there is accumulation, causing poisoning and convulsions.
- Medication of the elderly should be adjusted the dose according to the needs and tolerability, and over the age of 70 should be halved.
- Prevent straying into the blood vessels when anaesthetizing, prevent local anesthetic poisoning.
- We should pay attention to monitoring blood pressure, electrocardiogram, and with rescue equipment when arrhythmia Treatment; drug administration should be immediately discontinued in some circumstances. For example, ECG P-R interval prolongs or QRS wave widens, other arrhythmia or the original arrhythmia deteriorates.
References
https://www.drugbank.ca/drugs/DB00281
https://en.wikipedia.org/wiki/Lidocaine
Chemical Properties
White to Off-White Solid
Originator
Xylocaine,Astra,US,1949
Uses
Apply to affected site 5 to 10 minutes before procedure. Duration of anesthesia is relatively short (<1 hour).
Uses
Local anesthesic;Na+ channel blocker
Uses
Anesthetic (local); antiarrhythmic (class IB). Long-acting, membrane stabilizing agent against ventricular arrhythmia. Originally developed as a local anesthetic.
Definition
ChEBI: Lidocaine hydrochloride is the anhydrous form of the hydrochloride salt of lidocaine. It functions as both a local anesthetic and cardiac depressant, and is commonly used as an antiarrhythmic agent. Its potency is higher and its effects last longer than those of procaine, but its duration of action is shorter than that of bupivacaine or prilocaine.
Manufacturing Process
One mol of 2,6-xylidine is dissolved in 800 ml glacial acetic acid. The mixture
is cooled to 10°C, after which 1.1 mol chloracetyl chloride is added at one
time. The mixture is stirred vigorously during a few moments after which
1,000 ml half-saturated sodium acetate solution, or other buffering or
alkalizing substance, is added at one time. The reaction mixture is shaken
during half an hour. The precipitate formed which consists of ω-chloro-2,6-
dimethyl-acetanilide is filtered off, washed with water and dried. The product
is sufficiently pure for further treatment. The yield amounts to 70 to 80% of
the theoretical amount.
One mole of the chloracetyl xylidide thus prepared and 2.5 to 3 mols diethyl
amine are dissolved in 1,000 ml dry benzene. The mixture is refluxed for 4 to
5 hours. The separated diethyl amine hydrochloride is filtered off. The benzene
solution is shaken out two times with 3N hydrochloric acid, the first time with
800 ml and the second time with 400 ml acid. To the combined acid extracts
is added an approximately 30% solution of sodium hydroxide until the
precipitate does not increase.
The precipitate, which sometimes is an oil, is taken up in ether. The ether
solution is dried with anhydrous potassium carbonate after which the ether is
driven off. The remaining crude substance is purified by vacuum distillation.
During the distillation practically the entire quantity of the substance is carried
over within a temperature interval of 1° to 2°C. The yield approaches the
theoretical amount. MP 68° to 69°C. BP 180° to 182°C at 4 mm Hg; 159° to
160°C at 2 mm Hg. (Procedure is from US Patent 2,441,498.)
brand name
Alpha caine Hydrochloride (Carlisle); Anestacon (Polymedica); Laryng-O-Jet (International Medication); Lidocaton (Phar maton); Lidopen (Meridian); Xylocaine (Abraxis); Xylo caine (AstraZeneca); Xylocaine (Dentsply).
Therapeutic Function
Local anesthetic, Antiarrhythmic
Biological Functions
Lidocaine hydrochloride (Xylocaine) is the most commonly used local anesthetic. It is well tolerated, and in addition to its use in infiltration and regional nerve blocks, it is commonly used for spinal and topical anesthesia and as an antiarrhythmic agent. Lidocaine has a more rapidly occurring, more intense, and more prolonged duration of action than does procaine.
General Description
Lidocaine hydrochloride,2-(diethylamino)-2 ,6 -acetoxylidide monohydrochloride(Xylocaine), was conceived as a derivative of gramine(3-dimethylaminomethylindole) and introduced as a localanesthetic. It is now being used intravenously as a standardparenteral agent for suppression of arrhythmias associatedwith acute myocardial infarction and cardiac surgery.It isthe drug of choice for the parenteral treatment of prematureventricular contractions.
Mechanism of action
Lidocaine can block Na+ and K+ ion channels and regulate intracellular and extracellular calcium concentrations through other ligand-gated ion channels. Lidocaine was the first sodium channel blocker to be identified. Its main mechanism of action is blocking voltage-gated Na+ channels (VGSC/NaVs).
Clinical Use
Lidocaine hydrochloride is a class IB antiarrhythmicagent with a different effect on the electrophysiologicalproperties of myocardial cells from that of procainamideand quinidine. It binds with equal affinity to the active (A)and inactive (I) Na+ ion channels. It depresses diastolic depolarizationand automaticity in the Purkinje fiber networkand increases the functional refractory period relative toaction potential duration, as do procainamide and quinidine.It differs from the latter two drugs, however, in that it doesnot decrease, and may even enhance, conduction velocity and increase membrane responsiveness to stimulation.There are fewer data available on the subcellular mechanismsresponsible for the antiarrhythmic actions of lidocainethan on the more established drug quinidine. It has been proposedthat lidocaine has little effect on membrane cation exchangeof the atria. Sodium ion entrance into ventricularcells during excitation is not influenced by lidocaine becauseit does not alter conduction velocity in this area.Lidocaine hydrochloride does depress Na+ influx duringdiastole, as do all other antiarrhythmic drugs, to diminishautomaticity in myocardial tissue. It also alters membraneresponsiveness in Purkinje fibers, allowing increased conductionvelocity and ample membrane potential at the timeof excitation.
Safety Profile
Poison by ingestion, intraperitoneal, intravenous, subcutaneous, intramuscular, and intratracheal routes. Human systemic effects: somnolence, respiratory depression, low blood pressure, cardiomyopathy includmg infarction, pulse rate increase. An experimental teratogen. Other experimental reproductive effects. A skin and eye irritant. An anesthetic. When heated to decomposition it emits very toxic fumes of NOx and HCl.
storage
Store in a tightly closed container at room temperature away from light and moisture. Do not store in the bathroom. Do not freeze. Keep all medications away from children and pets.
Lidocaine hydrochloride Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Guanlang Biotechnology Co,.LTD | +8619930503252 | daisy@crovellbio.com | China | 5964 | 58 |
| Shanghai Worldyang Chemical Co.,Ltd. | ,+86-21-56795779; +8613651600618 | sales@worldyachem.com | China | 879 | 58 |
| Hebei Lingding Biotechnology Co., Ltd. | +86-18031140164 +86-19933155420 | erin@hbldbiotech.com | China | 878 | 58 |
| Nanjing Deda New Material Technology Co., Ltd | +8613223293093 | bella@njdeda.com | China | 81 | 58 |
| Ouhuang Engineering Materials (Hubei) Co., Ltd | +8617702722807 | admin@hbouhuang.com | China | 2259 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12456 | 58 |
| Shanghai UCHEM Inc. | +862156762820 +86-13564624040 | sales@myuchem.com | China | 6710 | 58 |
| Anhui Yiao New Material Technology Co., Ltd | +86-199-55145978 +8619955145978 | sales8@anhuiyiao.com | China | 253 | 58 |
| Henan Tengmao Chemical Technology Co. LTD | +8615238638457 | salesvip2@hntmhg.com | China | 415 | 58 |
| Xiamen Wonderful Bio Technology Co., Ltd. | +8613043004613 | Sara@xmwonderfulbio.com | China | 305 | 58 |
Related articles
- Lidocaine hydrochloride for Chronic Pain Treatment
- Lidocaine hydrochloride effectively reduces non-specific chronic pain, neuropathic pain, musculoskeletal pain and improves qua....
- Jan 25,2024
- Lidocaine hydrochloride: mechanism of action, pharmacokinetics and activities
- Lidocaine hydrochloride is a medication that works by blocking sodium channels in neuronal cell membranes, preventing the init....
- Jun 28,2023
- Lidocaine Hcl: Uses, Side effects, Overdose, Interactions
- Lidocaine hydrochloride was synthesized by L?fgren and Lundquist in 1943, and was clinically introduced in 1948. It remains on....
- Sep 16,2021
View Lastest Price from Lidocaine hydrochloride manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-26 | Lidocaine hydrochloride
73-78-9
|
US $1.00-1.00 / kg | 1kg | 99% | 10 tons | Dorne Chemical Technology co. LTD | |
 |
2024-04-26 | Lidocaine hydrochloride
73-78-9
|
US $20.00-10.00 / kg | 1kg | 98% | 20 | Hebei Kangcang new material Technology Co., LTD | |
 |
2024-04-26 | Lidocaine HCL
73-78-9
|
US $5.00-1.00 / kg | 1kg | 99.5 | 10 ton per month | Nanjing Deda New Material Technology Co., Ltd |
-

- Lidocaine hydrochloride
73-78-9
- US $1.00-1.00 / kg
- 99%
- Dorne Chemical Technology co. LTD
-

- Lidocaine hydrochloride
73-78-9
- US $20.00-10.00 / kg
- 98%
- Hebei Kangcang new material Technology Co., LTD
-

- Lidocaine HCL
73-78-9
- US $5.00-1.00 / kg
- 99.5
- Nanjing Deda New Material Technology Co., Ltd