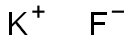Potassium fluoride
- CAS No.
- 7789-23-3
- Chemical Name:
- Potassium fluoride
- Synonyms
- KF;Fluorure de potassium;Potassiumfluoride,anhydrous;potassium fluoride spray dried;SDKF;DG 18;Potassium fL;MANNITOL SALT;Kaliumfluorid;SUCROSE, MOLECUL
- CBNumber:
- CB4237549
- Molecular Formula:
- FK
- Molecular Weight:
- 58.1
- MDL Number:
- MFCD00011398
- MOL File:
- 7789-23-3.mol
- MSDS File:
- SDS
| Melting point | 858 °C (lit.) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Boiling point | 1505 °C | ||||||||||||||
| Density | 2.48 | ||||||||||||||
| vapor density | 2 (vs air) | ||||||||||||||
| vapor pressure | 1.3 hPa (885 °C) | ||||||||||||||
| refractive index | 1.363 | ||||||||||||||
| Flash point | 1505°C | ||||||||||||||
| storage temp. | Store at +5°C to +30°C. | ||||||||||||||
| solubility | H2O: 1 M at 20 °C, clear, colorless | ||||||||||||||
| form | spray-dried | ||||||||||||||
| Specific Gravity | 2.481 | ||||||||||||||
| color | White | ||||||||||||||
| PH | 7.0-8.5 (25℃, 1M in H2O) | ||||||||||||||
| Odor | Odorless | ||||||||||||||
| Water Solubility | 92.3 g/100 mL (18 ºC) | ||||||||||||||
| Sensitive | Hygroscopic | ||||||||||||||
| λmax |
λ: 260 nm Amax: 0.01 λ: 280 nm Amax: 0.01 |
||||||||||||||
| Crystal Structure | NaCl type | ||||||||||||||
| crystal system | Cube | ||||||||||||||
| Merck | 14,7632 | ||||||||||||||
| BRN | 3902818 | ||||||||||||||
| Space group | Fm3m | ||||||||||||||
| Lattice constant |
|
||||||||||||||
| Exposure limits |
ACGIH: TWA 2.5 mg/m3 NIOSH: IDLH 250 mg/m3; TWA 2.5 mg/m3 |
||||||||||||||
| Stability | Stable. Protect from moisture. Incompatible with strong acids, strong bases. | ||||||||||||||
| InChIKey | NROKBHXJSPEDAR-UHFFFAOYSA-M | ||||||||||||||
| LogP | -0.77 at 25℃ | ||||||||||||||
| CAS DataBase Reference | 7789-23-3(CAS DataBase Reference) | ||||||||||||||
| EWG's Food Scores | 1-3 | ||||||||||||||
| FDA UNII | 9082WG1G3F | ||||||||||||||
| NIST Chemistry Reference | Potassium fluoride(7789-23-3) | ||||||||||||||
| EPA Substance Registry System | Potassium fluoride (7789-23-3) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS05,GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301+H311+H331-H318 | |||||||||
| Precautionary statements | P261-P280-P301+P310-P302+P352+P312-P304+P340+P311-P305+P351+P338 | |||||||||
| Hazard Codes | T | |||||||||
| Risk Statements | 25-23/24/25 | |||||||||
| Safety Statements | 45-26-36/37 | |||||||||
| RIDADR | UN 1812 6.1/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | TT0700000 | |||||||||
| F | 3 | |||||||||
| Hazard Note | Harmful | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 2826 19 90 | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | III | |||||||||
| Toxicity | MLD in guinea pigs (mg/kg): 250 orally, 350 s.c.; in frogs (mg/kg): 375 s.c. (Simonin) | |||||||||
| NFPA 704 |
|
Potassium fluoride price More Price(85)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | PX1467 | Potassium Fluoride Meets ACS Specifications, Meets Reagent Specifications for testing USP/NF monographs GR ACS | 7789-23-3 | 12kg | $1150 | 2024-03-01 | Buy |
| Sigma-Aldrich | NIST2203 | Potassium fluoride NIST? SRM? 2203, standard for ion-selective electrodes | 7789-23-3 | 125g | $807 | 2024-03-01 | Buy |
| Sigma-Aldrich | 60238 | Potassium fluoride BioUltra, ≥99.5% (F) | 7789-23-3 | 100G | $88.7 | 2024-03-01 | Buy |
| Sigma-Aldrich | 60238 | Potassium fluoride BioUltra, ≥99.5% (F) | 7789-23-3 | 500G | $352 | 2024-03-01 | Buy |
| Sigma-Aldrich | 229814 | Potassium fluoride ≥99.97% trace metals basis | 7789-23-3 | 10g | $140 | 2024-03-01 | Buy |
Potassium fluoride Chemical Properties,Uses,Production
Chemical Properties
Potassium fluoride, KF, is a colorless, deliquescent crystalline solid that has a melting point of 846 °C(1550 °F). Potassium fluoride has a salty taste and is poisonous. It is soluble in water,but insoluble in alcohol. Potassium fluoride is used in etching glass,preservatives, and insecticides.
Physical and Chemical Properties
Potassium fluoride is basis raw material of manufacturing fluoride, chemical formula is KF. Molecular weight is 58.10. It is colorless cubic crystal or white powder. It is poisonous! It is deliquescent. Taste is salty. Specific gravity is 2.48. Melting point is 858℃. Boiling point is 1505℃. It is soluble in water. It was dissolved in hydrofluoric acid and ammonia. It is insoluble in ethanol, acetone. Aqueous solution is alkaline and it can corrode glass and porcelain. It can cause irritation for human skin, mucous membrane and eye. When belows 40.2℃, it can crystallize in an aqueous solution to give dihydrate KF·2H2O, which is monoclinic, it is self-dissolved crystalline water at 41℃. It can be obtained by the thermal decomposition of potassium hydrogen fluoride or potassium carbonate (or potassium hydroxide) and hydrofluoric acid (40% or anhydrous). It can be used for glass engraving, food preservation, it can be also used as welding flux, fluoride, pesticides,etc.

Figure 1 the structural formula of potassium fluoride.
Uses
Anhydrous Potassium fluoride is used in organic synthesis as a catalyst for various reactions, or to introduce fluorine into organic molecules. For example, fluoro compounds can be prepared by replacing labile chlorine atoms by fluorine atoms, as in the manufacture of sodium fluoroacetate, a rat poison. The nucleophilic strength of F− and the solubility of KF in aprotic organic solvents may be improved by using crown ethers. The “naked” fluoride ion obtained is an efficient fluorinating agent.
Preparation
Potassium fluoride is prepared by reacting potassium carbonate (or KOH) with aqueous hydrofluoric acid. Care is necessary in handling the anhydrous salt, to prevent its hydration.
Chemical Properties
White, crystalline, deliquescent powder; sharp saline taste.Soluble in water and hydrogen fluoride, insoluble in alcohol.
Chemical Properties
Potassium fluoride is a white crystalline solid.
Uses
Potassium fluoride is used in metal finishing, batteries, coatings and photographic chemicals. It is utilized for the study of ion-specific swelling and de-swelling of ampholytic polymer gels as well as in the measurement of electronic polarizabilities of ions in polymers of alkali halides. It finds application in the electronic industry as a metal surface treatment product. It is used as a preservative, a food additive, a catalyzer and a water absorbing agent. In the Finkelstein reaction, it is actively involved in the conversion of chlorocarbons to fluorocarbons using polar solvents like dimethyl formamide and ethylene glycol.
Uses
Definition
ChEBI: Potassium fluoride is a fluoride salt having K+ as the counterion. It has a role as a poison. It is a fluoride salt and a potassium salt.
Reactivity Profile
Potassium fluoride reacts with acids to evolve corrosive and toxic hydrogen fluoride. Aqueous solutions corrode glass and consequently are prepared and stored in polyethylene containers. The pure solid may be stored in glass containers. Reacts violently with (Pt + BrF3). [NTP 1992].
Hazard
Toxic by ingestion and inhalation, strong irritant to tissue.
Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Safety Profile
Poison by ingestion and intraperitoneal routes. Moderately toxic by subcutaneous route. Experimental teratogenic effects. A corrosive irritant to the eyes, skin, and mucous membranes. Mutation data reported. A very reactive material. When heated to decomposition it emits toxic fumes of K2O and F-. Used in etchmg glass, as a presewative, as an insecticide, and in organic synthesis. See also FLUORIDES and HYDROFLUORIC ACID.
Potential Exposure
Potassium fluoride is used in etching glass; as a preservative and insecticide.
Shipping
UN1812 Potassium fluoride, solid, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Incompatibilities
Incompatible with strong acids; reacts releasing hydrogen fluoride. Aqueous solutions corrode glass and consequently are prepared and stored in polyeth- ylene containers. The pure solid may be stored in glass containers. Reacts violently with (Pt 1 BrF 3 )
Potassium fluoride Preparation Products And Raw materials
Raw materials
Preparation Products
1of7
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12446 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21667 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | fandachem@gmail.com | China | 9355 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32836 | 60 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +8618949832763 | info@tnjchem.com | China | 2989 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29888 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8820 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | sales@amoychem.com | China | 6387 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
View Lastest Price from Potassium fluoride manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-09-13 | Potassium fluoride
7789-23-3
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mojin Biotechnology Co., Ltd | |
 |
2023-07-19 | potassium fluoride
7789-23-3
|
US $30.00 / KG | 1KG | 99% | 20 Tons | Wuhan wingroup Pharmaceutical Co., Ltd | |
 |
2023-03-27 | Potassium fluoride
7789-23-3
|
US $20.00 / KG | 1KG | 99.9% | 500MT/month | XINGTAI XINGJIU NEW MATERIAL TECHNOLOGY CO., LTD |
-

- Potassium fluoride
7789-23-3
- US $0.00 / KG
- 99%
- Hebei Mojin Biotechnology Co., Ltd
-

- potassium fluoride
7789-23-3
- US $30.00 / KG
- 99%
- Wuhan wingroup Pharmaceutical Co., Ltd
-

- Potassium fluoride
7789-23-3
- US $20.00 / KG
- 99.9%
- XINGTAI XINGJIU NEW MATERIAL TECHNOLOGY CO., LTD