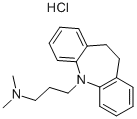
Imipramine hydrochloride
- CAS No.
- 113-52-0
- Chemical Name:
- Imipramine hydrochloride
- Synonyms
- g22355;imidol;ia-pram;imavate;iprogen;tofranil;deprinol;dynazina;efuranol;fujisawa
- CBNumber:
- CB6698241
- Molecular Formula:
- C19H25ClN2
- Molecular Weight:
- 316.87
- MDL Number:
- MFCD00012669
- MOL File:
- 113-52-0.mol
- MSDS File:
- SDS
| Melting point | 168-1700C |
|---|---|
| Flash point | 9℃ |
| storage temp. | 2-8°C |
| solubility | H2O: 50 mg/mL |
| form | crystalline |
| color | white |
| PH | 4.2~5.2(100g/l,25℃) |
| Water Solubility | Soluble in water |
| λmax | 260nm(lit.) |
| Merck | 14,4920 |
| CAS DataBase Reference | 113-52-0(CAS DataBase Reference) |
| FDA UNII | BKE5Q1J60U |
| EPA Substance Registry System | 5H-Dibenz[b,f]azepine-5-propanamine, 10,11-dihydro-N,N-dimethyl-, monohydrochloride (113-52-0) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H300 | |||||||||
| Precautionary statements | P264-P270-P301+P310-P405-P501 | |||||||||
| Hazard Codes | Xn,T,F | |||||||||
| Risk Statements | 23/25-36/38-36/37/38-22-39/23/24/25-23/24/25-11 | |||||||||
| Safety Statements | 7-16-24-33-45-36-26-36/37 | |||||||||
| RIDADR | UN 1230 3/PG 2 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | HO1925000 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 2933995800 | |||||||||
| Toxicity | LD50 in mice, rats (mg/kg): 400, 490 orally; 110, 90 i.p. (Tobe) | |||||||||
| NFPA 704 |
|
Imipramine hydrochloride price More Price(38)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | I-902 | Imipramine hydrochloride solution 1.0?mg/mL in methanol (as free base), ampule of 1?mL, certified reference material, Cerilliant? | 113-52-0 | 1mL | $27.7 | 2024-03-01 | Buy |
| Sigma-Aldrich | I0899 | Imipramine hydrochloride BioXtra, ≥99% (TLC) | 113-52-0 | 5g | $107 | 2024-03-01 | Buy |
| Sigma-Aldrich | BP932 | Imipramine hydrochloride British Pharmacopoeia (BP) Reference Standard | 113-52-0 | 50MG | $229 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1338007 | Imipramine hydrochloride United States Pharmacopeia (USP) Reference Standard | 113-52-0 | 200mg | $436 | 2024-03-01 | Buy |
| TCI Chemical | I0971 | Imipramine Hydrochloride >98.0%(HPLC) | 113-52-0 | 1g | $43 | 2024-03-01 | Buy |
Imipramine hydrochloride Chemical Properties,Uses,Production
Chemical Properties
White Solid
Originator
Tofranil,Ciba Geigy,France,1959
Uses
Imipramine hydrochloride is used as a serotonin uptake inhibitor. It mainly used in the treatment of major depression and enuresis (inability to control urination). It has also been evaluated for use in panic disorder.
Uses
Antidepressant;5-HT transport inhibitor
Uses
Tricyclic antidepressant; inhibits the serotonin and norepinephrine transporters. Has little effect on the dopamine transporter
Manufacturing Process
20 parts of imino dibenzyl are dissolved in 100 parts by volume of absolutely
dry benzene. A suspension of 4 parts NaNH2 in 50 parts by volume of
absolute benzene are then added dropwise at 50° to 60°C after which the
mixture is boiled for an hour under reflux. 13 parts of 3-dimethylamino n_x0002_propyl chloride are then added dropwise at 40° to 50°C and the mixture is
boiled for 10 hours under reflux. After cooling, the benzene solution is
thoroughly washed with water, whereupon the basic constituents are extracted
with dilute hydrochloric acid.
The hydrochloric extract is then made alkaline and the separated base is
extracted with ether. After drying, the solvent is evaporated and the residue is
distilled in the high vacuum, whereby the N-(3-dimethylaminopropyl)-imino
dibenzyl passes over at a temperature of 160°C under 0.1 mm pressure. The
chlorohydrate with a melting point of 174° to 175°C is obtained therefrom
with alcoholic hydrochloric acid.
brand name
Janimine (Abbott); Pramine (Alra); Presamine (Sanofi Aventis); Tofranil (Novartis); Tofranil (Tyco).
Therapeutic Function
Antidepressant
General Description
Imipramine hydrochloride, 5-[3-(dimethylamino)propyl]-10,11-dihydro-5H-dibenz[b,f]azepine monohydrochloride(Tofranil), is the lead compound of the TCAs. It is also a closerelative of the antipsychotic phenothiazines (replace the10–11 bridge with sulfur, and the compound is the antipsychoticagent promazine). It has weaker D2 postsynaptic blockingactivity than promazine and mainly affects amines (5-HT,NE, and DA) via the transporters. As is typical of dimethylaminocompounds, anticholinergic and sedative (central H1block) effects tend to be marked. The compound per se has a tendency toward a high 5-HT-to-NE uptake block ratio andprobably can be called a serotonin transport inhibitor(SERTI). Metabolic inactivation proceeds mainly by oxidativehydroxylation in the 2-position, followed by conjugationwith glucuronic acid of the conjugate. Urinary excretion predominates(about 75%), but some biliary excretion (up to25%) can occur, probably because of the large nonpolargrouping. Oxidative hydroxylation is not as rapid or completeas that of the more nucleophilic ring phenothiazine antipsychotics;consequently, appreciable N-demethylation occurs,with a buildup of norimipramine (or desimipramine).
Biological Activity
imipramine (hydrochloride) is a tricyclic antidepressant and is mainly used in the treatment of major depression and enuresis [1].antidepressants are antagonists of many neurotransmitter receptors in human brain [3].imipramine is the first tricyclic antidepressant that acts mainly as an inhibitor of serotonin and norepinephrine transporters [2]. in radioligand binding assays, imipramine inhibited serotonin and norepinephrine transporters with kd values of 1.4 and 37 nm, respectively [2]. imipramine is also inhibited histamine h1 receptor, muscarinic acetylcholine receptor and α1-adrenergic receptor with kd values of 37, 46, and 32 nm, respectively [4].in rodents, imipramine abolished the depressive syndrome produced by the acute administration of reserpine. imipramine also possessed central anticholinergic activity and attenuate the activity of the centrally acting muscarinic agents tremorine and oxotremorine. imipramine inhibited the presynaptic uptake of na and 5-ht, and relatively weak against da [1].
Biochem/physiol Actions
Tricyclic antidepressant; inhibits the serotonin and norepinephrine transporters with Kis of 7.7 nM and 67 nM, respectively. Has little effect on the dopamine transporter (Ki = 25 μM).
Clinical Use
The demethylated metabolite is less anticholinergic, lesssedative, and more stimulatory and is a SNERI.Consequently, a patient treated with imipramine has twocompounds that contribute to activity. Overall, the effect isnonselective 5-HT versus NE reuptake.
Safety Profile
Human poison by ingestion. An experimental poison by ingestion, intravenous, subcutaneous, and intraperitoneal routes. An experimental teratogen. Human systemic effects by ingestion: sleep, somnolence, convulsions, muscle contraction or spasticity, coma, blood pressure decrease, dyspnea (difficulty in breathing), paresthesia (abnormal sensations), and kidney changes. Experimental reproductive effects. Mutation data reported. Used in the treatment of depression. When heated to decomposition it emits very toxic fumes of NO, and HCl. See also DIAZEPAM.
Veterinary Drugs and Treatments
In dogs and cats, imipramine has been used to treat cataplexy and urinary incontinence. In horses, imipramine has been used to treat narcolepsy and ejaculatory dysfunction (no parenteral dosage forms available).
Drug interactions
Potentially hazardous interactions with other drugs
Alcohol: increased sedative effect.
Analgesics: increased risk of CNS toxicity with
tramadol; possibly increased risk of side effects with
nefopam; possibly increased sedative effects with
opioids.
Anti-arrhythmics: increased risk of ventricular
arrhythmias with amiodarone - avoid; increased
risk of ventricular arrhythmias with disopyramide,
flecainide or propafenone; avoid with dronedarone.
Antibacterials: increased risk of ventricular
arrhythmias with delamanid, moxifloxacin and
possibly telithromycin - avoid with delamanid and
moxifloxacin.
Anticoagulants: may alter anticoagulant effect of
coumarins.
Antidepressants: enhanced CNS excitation and
hypertension with MAOIs and moclobemide -
avoid; concentration possibly increased with SSRIs;
risk of ventricular arrhythmias with citalopram
and escitalopram - avoid; possible increased risk of
convulsions with vortioxetine.
Antiepileptics: convulsive threshold lowered;
concentration reduced by carbamazepine,
phenobarbital and possibly fosphenytoin, phenytoin
and primidone.
Antimalarials: avoid with artemether/lumefantrine
and piperaquine with artenimol.
Antipsychotics: increased risk of ventricular
arrhythmias especially with droperidol, fluphenazine,
haloperidol, pimozide, sulpiride and zuclopenthixol
- avoid; increased antimuscarinic effects with
clozapine and phenothiazines; concentration
increased by antipsychotics.
Antivirals: increased risk of ventricular arrhythmias
with saquinavir - avoid; concentration possibly
increased with ritonavir.
Atomoxetine: increased risk of ventricular
arrhythmias and possibly convulsions.
Beta-blockers: increased risk of ventricular
arrhythmias with sotalol; concentration increased by
labetalol and propranolol
.
Clonidine: tricyclics antagonise hypotensive
effect; increased risk of hypertension on clonidine
withdrawal.
Dapoxetine: possibly increased risk of serotonergic
effects - avoid.
Dopaminergics: avoid use with entacapone; CNS
toxicity reported with selegiline and rasagiline.
Pentamidine: increased risk of ventricular
arrhythmias.
Sympathomimetics: increased risk of hypertension
and arrhythmias with adrenaline and noradrenaline;
metabolism possibly inhibited by methylphenidate.
Metabolism
Imipramine is extensively demethylated by first-pass
metabolism in the liver, to its primary active metabolite,
desipramine (desmethylimipramine). Paths of
metabolism of both imipramine and desipramine include
hydroxylation and N-oxidation.
About 80% is excreted in the urine and about 20% in the
faeces, mainly in the form of inactive metabolites. Urinary
excretion of unchanged imipramine and of the active
metabolite desipramine is about 5% and 6% respectively.
Only small quantities of these are excreted in the faeces.
storage
Store at -20°C
References
[1]. spencer ps. review of the pharmacology of existing antidepressants. br j clin pharmacol. 1977;4suppl 2:57s-68s.
[2]. tatsumi m, groshan k, blakely rd, et al. pharmacological profile of antidepressants and related compounds at human monoamine transporters. eur j pharmacol. 1997 dec 11;340(2-3):249-58.
[3]. cusack b, nelson a, richelson e. binding of antidepressants to human brain receptors: focus on newer generation compounds. psychopharmacology (berl). 1994 may;114(4):559-65.
Imipramine hydrochloride Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49390 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | sales@tnjchem.com | China | 34572 | 58 |
| HANGZHOU CLAP TECHNOLOGY CO.,LTD | 86-571-88216897,88216896 13588875226 | sales@hzclap.com | CHINA | 6313 | 58 |
| ANHUI WITOP BIOTECH CO., LTD | +8615255079626 | eric@witopchemical.com | China | 23556 | 58 |
| Xi'an MC Biotech, Co., Ltd. | 029-89275612 +8618991951683 | mcbio_sales@163.com | China | 2255 | 58 |
| AFINE CHEMICALS LIMITED | 0571-85134551 | info@afinechem.com | CHINA | 15377 | 58 |
View Lastest Price from Imipramine hydrochloride manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-04-27 | Imipramine hydrochloride
113-52-0
|
US $67.00 / kg | 1kg | 99% | 5000 tons | Hebei Duling International Trade Co. LTD | |
 |
2022-10-11 | Imipramine hydrochloride
113-52-0
|
US $0.00-0.00 / KG | 1KG | 98% | 1ton | Henan Aochuang Chemical Co.,Ltd. | |
 |
2019-07-06 | Imipramine hydrochloride
113-52-0
|
US $1.00 / KG | 1G | 98% | 100KG | Career Henan Chemical Co |
-

- Imipramine hydrochloride
113-52-0
- US $67.00 / kg
- 99%
- Hebei Duling International Trade Co. LTD
-

- Imipramine hydrochloride
113-52-0
- US $0.00-0.00 / KG
- 98%
- Henan Aochuang Chemical Co.,Ltd.
-

- Imipramine hydrochloride
113-52-0
- US $1.00 / KG
- 98%
- Career Henan Chemical Co