Adapalene
- CAS No.
- 106685-40-9
- Chemical Name:
- Adapalene
- Synonyms
- Adapalen;Differin;6-(3-(AdaMantan-1-yl)-4-Methoxyphenyl)-2-naphthoic acid;6-[3-(1-ADAMANTYL)-4-METHOXYPHENYL]-2-NAPHTHALENE CARBOXYLIC ACID;6-[3-(1-adamantyl)-4-methoxy-phenyl]naphthalene-2-carboxylic acid;ADPL;CD-271;Differi;p75(NTR);Adaparin
- CBNumber:
- CB6771245
- Molecular Formula:
- C28H28O3
- Molecular Weight:
- 412.52
- MDL Number:
- MFCD03106112
- MOL File:
- 106685-40-9.mol
- MSDS File:
- SDS
| Melting point | 319-3220C |
|---|---|
| Boiling point | 606.3±55.0 °C(Predicted) |
| Density | 1.233±0.06 g/cm3(Predicted) |
| RTECS | QJ1987000 |
| storage temp. | 2-8°C |
| solubility | DMSO: >10mg/mL |
| pka | 4.2(at 25℃) |
| form | White solid |
| color | white to off-white |
| Merck | 14,150 |
| Stability | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or DMF may be stored at -20°C for up to 3 months |
| InChIKey | LZCDAPDGXCYOEH-UHFFFAOYSA-N |
| SMILES | C1=C2C(C=C(C3=CC=C(OC)C(C45CC6CC(CC(C6)C4)C5)=C3)C=C2)=CC=C1C(O)=O |
| CAS DataBase Reference | 106685-40-9(CAS DataBase Reference) |
| FDA UNII | 1L4806J2QF |
| NCI Drug Dictionary | adapalene |
| ATC code | D10AD03,D10AD53 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H361d | |||||||||
| Precautionary statements | P201-P202-P280-P308+P313-P405-P501 | |||||||||
| WGK Germany | nwg | |||||||||
| HS Code | 2918992090 | |||||||||
| NFPA 704 |
|
Adapalene price More Price(68)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 114825-M | Adapalene | 106685-40-9 | 25mg | $155 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1011709 | Adapalene United States Pharmacopeia (USP) Reference Standard | 106685-40-9 | 200mg | $436 | 2024-03-01 | Buy |
| TCI Chemical | A2549 | Adapalene >98.0%(HPLC)(T) | 106685-40-9 | 1g | $364 | 2024-03-01 | Buy |
| TCI Chemical | A2549 | Adapalene >98.0%(HPLC)(T) | 106685-40-9 | 200mg | $104 | 2024-03-01 | Buy |
| Alfa Aesar | J67025 | Adapalene | 106685-40-9 | 25mg | $117 | 2021-12-16 | Buy |
Adapalene Chemical Properties,Uses,Production
Naphthoic acid derivative
Adapalene, a new class of drugs Naphthoic acid derivatives, was developed by France Galdeama Laboratories Company. It first entered into market in June 1996, under the trade name "Differin", mainly used for relieving inflammatory skin lesions, adjusting differentiation of hair follicles, gland epithelial cells, and also acne treatment.
Pharmacological effects
Adapalene is a retinoid compounds, and is proven to have anti-inflammatory properties in both in vivo and in vitro models of inflammation. It has stable chemical structure, and is not easily to break down in the air and the light. It shares the same mode of action with retinoic acid, bounding to specific retinoic acid nuclear receptors except that adapalene doesn’t bound to the cytoplasmic protein binding receptor. It has been proven to treat acne and affect acne vulgaris and differentiation in skin drug tests established by mouse animal model. Its mechanism of action is reducing the formation of micro-acne through leading the normal differentiation of hair follicle cells. In the standard anti-inflammatory assay both in vivo and in vitro, adapalene has a better effect than retinoic acid. It can inhibit the chemotaxis reaction of human polymorphonuclear leukocytes and also inhibit the metabolism of polymorphonuclear cell through inhibition the formation of anti-inflammatory mediators via oxidation reaction of arachidonic acid, thus relieving inflammatory response by the cell-mediated response. It is useful in the treatment of routine skin with acne, pimples and pustules as the main performance. It can also be used for the treatment of the acne presented in face, chest and back.
The above information is edited by the Chemicalbook of Dai Xiongfeng.
Pharmacokinetics
The transdermal absorption rate of adapalene is very low. In clinical trials, the adapalene level of skin acne areas subject to long-term treatment is undetectable. Appling C14 labeled adapalene for rats (intravenous, intraperitoneal injection, oral and dermal medication), rabbits (intravenous oral and dermal administration), respectively, clarified the distribution of radioactivity among various tissues. Also, liver, spleen adrenal and ovarian have the highest level. Adapalene is mainly metabolized through demethylation, hydroxylation and binding reactions of oxygen 1 and metabolism in vivo and mainly excreted through bile.
[Interaction] No interaction between the product and other skin drugs has been reported. But we should not use other retinoids or other drugs with similar mechanism of action. Adapalene has stable chemical structure, and is not easily to break down in the air and sunlight. A wide range of animal and human studies found no phototoxicity and photosensitivity. However, it is not clear whether it is safe to take it when animal and humans are subject to repeated exposure to sunlight or ultraviolet. Avoid excessive sunshine and UV radiation when use this product. Adapalene has a very low level of transdermal absorption, making it impossible to interact with the systematic medication drugs. No evidence was found that the effect of birth control pills, antibiotics and other oral medications are affected by the using Duff Man for treating skin disease.
Duff Man may have a slight local stimulation. Being treated together with the peeling agent, agent or contraction irritating substances can cause additional irritation. Therefore, use other skin medication early in the morning such as erythromycin (concentrations ≤4%), clindamycin phosphate (1%) aqueous solution or benzoyl peroxide gel (concentration ≤10%); Use Duff Man gel at night so that you can avoid drug co-degradation or accumulation of stimulation effect.
[Adverse reactions] During the first 2-4 weeks of treatment, the most common adverse reactions are erythema, dryness, scaling, itching, burning or tingling. The extent is mostly mild to moderate. Less frequent adverse reactions include: sunburn, skin irritation, burning and stinging skin discomfort. Rarely adverse reactions include: redness of acne, dermatitis and contact dermatitis, eye swelling, conjunctivitis, redness, itching, skin discoloration, rashes and eczema. Reduce the amount of drugs applied or totally withdrawal when serious adverse reactions happen.
Adapalene acid drug safety compared with other topical Vitamin A
The effect of traditional topical Vitamin A acid drugs (RA) on the treatment of acne vulgaris is indeed very good, but the irritating side effects limit its use. RA causes local irritation because of its unstable structure and poor receptor selectivity caused by series of weak divalent bond chains in its molecular structure. Studies have shown that RA can be degraded by a number of factors: exposure to sunlight within 24 h, 60.0%~80.0% of baseline concentration RA degradation, and if use an oxidizing agent such as benzoyl peroxide the same time when apply RA, 80% of RA would be degraded within 4 h, and all RA disappear within 24 h. In order to find a more stable molecule with similar effect as RA but much smaller side effect, people developed a naphthoic acid derivative-adapalene. Adapalene has small irritation effect due to: 1. Molecularly stable: The unstable divalent bond chains in RA are replaced by the aromatic ring of naphthoic acid, making it stable in the light and oxidants without degradation even in 72 h. 2. Highly selective receptor binding: after entering the cell, RA first bound to cytoplasmic vitamin A acid bound protein (CRABP), and has no selectivity when binds to nuclear receptor RARα, β, γ. Adapalene does not bind CRABP, but selectively binds to specific amino acids sequences of the binding site in nuclear receptor RAR-β and RAR-γ receptor, then binds to RXR, further binds to specific DNA sites, regulating transcription machinery, protein synthesis, and cell proliferation and differentiation. Therefore, it is highly specific and has a small side effect. 3. Low cellular toxicity: Study found that effects of different vitamin A acid on keratinocyte cell cytotoxicity are also different. Adapalene molecule is neutral, has low cytotoxicity to keratinocytes compared with the long-chain organic acids, Vitamin A acid. 4. Adapalene has a clear anti-inflammatory effect. However, this kind of effect for other Vitamin A acid is not still clear.
In short, adapalene, a third generation vitamin A drugs, not only have a stronger effect on regulation of epidermal cell differentiation, inhibit proliferation of hair follicle keratinocytes and keratinocytes, inhibition sebaceous cell proliferation, dissolved angle plug acne, etc than all-trans vitamin A acid drugs, but also has a strong anti-inflammatory effect. Because of its unique stability, its efficacy and tolerability is much higher than other Vitamin A acid drugs.
Uses
Retinoic acid analogs. Adapalene is an agonist of RARβ, and RARγ receptor. Adapalene inhibits cell proliferation and inducing apoptosis in colorectal cancer cells in vitro. Adapalene gel is an effective medication for treatment of acne.
Description
Adapalene is a synthetic retinoid and an agonist of retinoic acid receptors (RARs; Kds = 1,100, 34, and 130 nM for RARα, RARβ, and RARγ, respectively). It inhibits growth and differentiation of sebocytes in a concentration-dependent manner in primary rat preputial cell culture. Adapalene (10 μM) completely inhibits the activity of soybean 15-lipoxygenase (15-LOX) in an enzyme assay and inhibits the 5- and 15-LOX pathways in human blood polymorphonuclear leukocytes (PMNs). It reduces the protein levels of toll-like receptor 2 (TLR2) and IL-10 in skin explants isolated from patients with acne and healthy controls in a concentration-dependent manner but increases the expression of the antigen-presenting protein CD1d in acne skin explants while decreasing it in control explants. It inhibits inflammation in rodent models of ear edema induced by arachidonic acid and carrageenan-induced paw edema. Adapalene (100 μM) also induces apoptosis and inhibits proliferation of CC-531, HT-29, and LoVo colon cancer cells and reduces tumor growth in a DLD-1 colon cancer nude mouse xenograft model in a dose-dependent manner. Formulations containing adapalene have been used in the treatment of acne vulgaris.
Chemical Properties
White Crystalline Solid
Originator
Adaferin ,Laboratoires Galderma ,France
Uses
inhibits serotonin and noradrenaline reuptake antidepressant antimigraine therapeutic
Uses
Retinoid selective for retinoic acid receptor (RAR) subtypes β and γ. Antiacne.
Uses
anesthetic
Uses
Adapalene has been used as a retinoic acid receptor (RAR) agonist to study its effects on inhibiting transcription of hepatitis B virus in covalently closed circular DNA (cccDNA). It has also been used as a component to culture mixed lymphocyte reactions (MLR) to study its effects on isolated macrophages.
Uses
Acnetreatment
Indications
Adapalene (Differin) is a polyaromatic retinoidlike compound that binds to specific retinoic acid nuclear receptors and is thought to normalize the differentiation of keratinocytes in the sebaceous acroinfundibulum. Adapelene is indicated for topical treatment of acne. Minor local irritation is a common, usually tolerable side effect. In contrast to other drugs of the retinoid group, adapalene has not been shown to be teratogenic in rodents. However, since adequate human studies are lacking, its use in pregnant women should be discouraged until further information is available.
Definition
ChEBI: A naphthoic acid that is CD437 in which the phenolic hydroxy group has been converted to its methyl ether.
Manufacturing Process
Preparation of 6-(3-(1-adamantyl)-4-methoxyphenyl)-2-naphthoic acid consist
of 4 steps.
1. 2-(1-Adamantyl)-4-bromophenol.
34.6 g (200 mmol) of p-bromophenol and 30.4 g (200 mmol) of 1-
adamantanol are dissolved in 100 ml of dichloromethane. To the resulting
solution there are slowly added 10 ml of concentrated sulfuric acid. The
mixture is stirred for 8 hours at ambient temperature, poured into water,
neutralized with sodium bicarbonate, extracted with methylehe chloride, dried
and evaporated. After recrystallization in isooctane 52.8 g of the expected
product are obtained. Yield - 86%. MP: 140°-141°C.
2. 2-(1-Adamantyl)-4-bromoanisole.
To suspension of sodium hydride (80% in oil, 4.32 g, 144 mmol) in 50 ml of
THF, there are slowly added while maintaining the temperature at 20°C, 36.8
g (120 mmol) of 2-(1-adamantyl)-4-bromophenol. The mixture is stirred for 1
hour at ambient temperature at which point 9 ml of methyl iodide are added.
The mixture is then stirred for 2 hours at 20°C, poured into water, extracted
with ether, dried and evaporated. The product is purified by passage through a
silica column (10x30), eluting with a mixture of hexane (90%) and
dichloromethane (10%). On evaporation, 26.2 g of a white solid are obtained.
Yield - 68%. MP: 138°-139°C.
3. Methyl ester of 6-(3-(1-adamantyl)-4-methoxyphenyl)-2-naphthoic acid.
To a suspension of magnesium (1.64 g, 67.5 mmol in 30 ml of THF, there is
added a solution of 1.4 g (4.5 mmol) of 2-(1-adamantyl)-4-bromoanisole and
0.39 ml of dibromoethane in 10 ml of THF. The mixture is stirred until the
reaction is initiated and then there is slowly added a solution of (40.8 mmol)
of 2-(1-adamantyl)-4-bromoanisole in 90 ml of THF. The mixture is refluxed
for 2 hours, and then cooled to 20°C. After that 6.2 g (45 mmol) of
anhydrous ZnCl2 are added. The mixture is stirred for 1 hour at 20°C at which
point 7.95 g (30 mmol) of methyl 6-bromo-2-naphthoate are added followed
by addition of 300 g of NiCl2/1,2-(diphenylphosphino)ethane-complex as the
catalyst. The mixture is stirred again for 2 hours at 20°C, poured into water,
extracted with CH2Cl2 dried and evaporated. The product is isolated by column chromatography, eluting with a mixture of heptane (70%) and
dichloromethane (30%) and then recrystallized in ethyl acetate. 12.2 g of the
expected product are obtained. Yield - 78%. MP: 222°-223°C.
4. 6-(3-(1-Adamantyl)-4-methoxyphenyl)-2-naphthoic acid.
10.5 g of the ester obtained above (step 3) are treated with a solution of soda
in methanol (200 ml, 4.2 N). The mixture is heated at reflux for 48 hours. The
solvents are evaporated and the resulting residue is taken up in water and
acidified with concentrated HCl. The solid is filtered and dried under vacuum
over phosphoric anhydride. The resulting white solid is recrystallized in a
mixture of THF and ethyl acetate. 8.2 g of expected product are obtained.
Yield - 81%. MP: 325°-327°C.
brand name
Differin (Galderma).
Therapeutic Function
Antiacne
General Description
Adapalene is a third-generation synthetic retinoid and a highly lipophilic compound derived from naphthoic acid. It is widely used in the treatment of acne.
Biological Activity
Retinoic acid analog that is a RAR β and RAR γ agonist (AC 50 values are 2.2, 9.3, 22 and > 1000 nM for RAR β , RAR γ , RAR α and RXR α receptors respectively). Inhibits proliferation and induces apoptosis in colorectal cancer cell in vitro . Displays comedolytic activity.
Biochem/physiol Actions
Retinoic acid analogue that is a RARβ and RARγ agonist (AC50 values are 2.2, 9.3, 22 and > 1000 nM for RARβ, RARγ, RARα and RXRα receptors respectively). Inhibits proliferation and induces apoptosis in colorectal cancer cells in vitro. Displays comedolytic activity. Its unique pharmacological properties make it superior to other retinoids for the treatment of acne.
storage
Store at +4°C
Structure and conformation
Retinoid-like compound that binds to retinoic acid nuclear receptors, but not to cytoplasmic receptor proteins.
References
1) Michel et al. (1998), Pharmacology of Adapalene; Br. J. Dermatol., 139 Suppl. 52 3 2) Ocker et al. (2003), The synthetic retinoid adapalene inhibits proliferation and induces apoptosis in colorectal cancer cells on vitro; Int. J. Cancer, 107 453 3) Milikan et al. (2000), Adapalene: an update on newer comparative studies between the various retinoids; Int. J. Dermatol., 39 784 4) Bernard et al. (1993), Adapalene, a new chemical entity with retinoid activity; Skin Pharmacol., 6 61
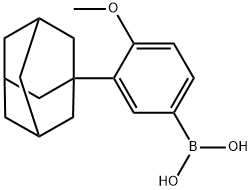
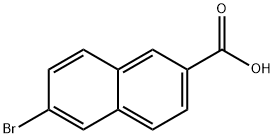
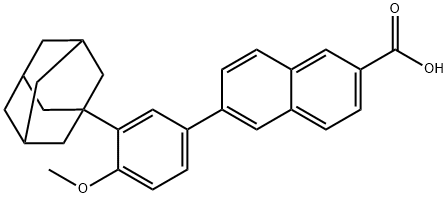
Adapalene Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Changsha Junyu Chemexpress.co., Ltd | +86-13723890100 | info@csjyyy.cn | China | 134 | 58 |
| Shaanxi Cuikang Pharmaceutical Technology Co., Ltd | +86-18791163155 +86-13119157289 | 13119157289@163.com | China | 2972 | 58 |
| BEIJING SJAR TECHNOLOGY DEVELOPMENT CO., LTD. | +86-18600796368 +86-18600796368 | sales@sjar-tech.com | China | 260 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-81148696 +86-15536356810 | 1022@dideu.com | China | 3878 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | deasea125996@gmail.com | China | 2503 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 20293 | 58 |
| Sigma Audley | +86-15937194204 +86-18126314766 | nova@sh-teruiop.com | China | 491 | 58 |
| Hebei Mojin Biotechnology Co.,Ltd | +86-15028179902 | angelia@hbmojin.com | China | 1069 | 58 |
| HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD | +8615383190639 | admin@86-ss.com | China | 1000 | 58 |
| Beijing Cooperate Pharmaceutical Co.,Ltd | 010-60279497 | sales01@cooperate-pharm.com | CHINA | 1811 | 55 |
Related articles
- Adapalene and tretinoin
- As a third-generation anti-inflammatory retinoid, topical adapalene is among the best-tolerated topical retinoids, including w....
- Aug 2,2024
View Lastest Price from Adapalene manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-09-23 | Adapalene
106685-40-9
|
US $1000.00-400.00 / kg | 1kg | 99% | 5000 | HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD | |
 |
2024-09-20 | Adapalene
106685-40-9
|
US $0.00 / g | 1g | More Than 99% | 50kg/Month | BEIJING SJAR TECHNOLOGY DEVELOPMENT CO., LTD. | |
 |
2024-09-18 | Adapalene
106685-40-9
|
US $0.00-0.00 / Kg/Bag | 1g | 98%min | 1000g | WUHAN FORTUNA CHEMICAL CO., LTD |





