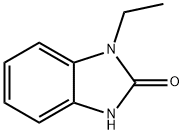
1-EBIO
- CAS No.
- 10045-45-1
- Chemical Name:
- 1-EBIO
- Synonyms
- EBIO;1-EB10;1-EBIO;Einecs 233-148-1;1-EthylbenziMidazolinone;1-ETHYL-2-BENZIMIDAZOLINONE;1-ethylbenzimidazolin-2-one;1-ETHYL-2-BENZCMIDAZOLINONE;1-Ethyl-2-benzimidazolineone;3-ethyl-1H-benzimidazol-2-one
- CBNumber:
- CB7146587
- Molecular Formula:
- C9H10N2O
- Molecular Weight:
- 162.19
- MDL Number:
- MFCD00005715
- MOL File:
- 10045-45-1.mol
| Melting point | 118-120 °C(Solv: ethyl acetate (141-78-6)) |
|---|---|
| Density | 1.161±0.06 g/cm3(Predicted) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | DMSO: ≥20mg/mL |
| form | powder |
| pka | 12.29±0.30(Predicted) |
| color | Crystalline |
| Stability | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 3 months. |
| FDA UNII | M82W79SS4W |
1-EBIO price More Price(44)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | SML0034 | 1-EBIO ≥98% (HPLC) | 10045-45-1 | 10mg | $100 | 2024-03-01 | Buy |
| Sigma-Aldrich | SML0034 | 1-EBIO ≥98% (HPLC) | 10045-45-1 | 50mg | $404 | 2024-03-01 | Buy |
| Cayman Chemical | 14575 | 1-EBIO ≥98% | 10045-45-1 | 5mg | $32 | 2024-03-01 | Buy |
| Cayman Chemical | 14575 | 1-EBIO ≥98% | 10045-45-1 | 10mg | $50 | 2024-03-01 | Buy |
| Cayman Chemical | 14575 | 1-EBIO ≥98% | 10045-45-1 | 50mg | $193 | 2024-03-01 | Buy |
1-EBIO Chemical Properties,Uses,Production
Description
1-EBIO is an activator of Ca2+-sensitive K+ channels (EC50 = 490 μM in T84 human carcinoma cells).This action is sensitive to the neurotoxin charybdotoxin (Ki = 3.5 nM). In mouse colonic epithelia, 1-EBIO also activates cAMP-sensitive K+ channels, a response this is inhibited by 293B.In this type of epithelium, but not mouse nasal epithelia, 1-EBIO activates both types of channels, resulting in large Cl- secretory currents.It also activates the human intermediate conductance Ca2+-activated K+ channel (EC50 = 74 μM).1-EBIO is currently used to study the role of these types of K+ channels in diverse physiological functions.
Uses
1-EBIO stimulates Cl- secretion in secretory epithelia through direct activation of clacium potassium channels.
1-EBIO was used to study the electrical activity modulated by Ca+2-activated K+ channels in rat central neurons. The effect of 1-EBIO on human intermediate conductance channels was studied.
The K+ channel opener 1-EBIO potentiates residual function of mutant CFTR in rectal biopsies from cystic fibrosis patients
Definition
ChEBI: 1-Ethyl-2-benzimidazolinone is a member of imidazoles.
Biological Activity
Activator of epithelial K Ca channels, stimulates a large and sustained trans-epithelial Cl - secretory response across T84 monolayers. Induces hyperpolarization to the same magnitude as ACh in aortic value endothelial cells.
storage
Room temperature
References
1) Adeagbo?et al.?(1999),?1-Ethyl-2-benzimidazolinone stimulates endothelial K(Ca) channels and nitric oxide formation in rat mesenteric vessels; Eur. J. Pharmacol.,?379?151 2) Orfila?et al. (2014),?Increasing small conductance Ca2+-activated potassium channel activity reverses ischemia-induced impairment of long-term potentiation; Eur. J. Neurosci.,?40?3179 3) Allen?et al.?(2011),?SK2 channels are neuroprotective for ischemia-induced neuronal cell death; J. Cereb. Blood Flow Metab.,?31?2302 4) Muller?et al. (2012),?Ca2+ activated K channels-new tools to induce cardiac commitment from pluripotent stem cells in mice and men; Stem Cell Rev.,?8?720 5) Fay?et al.?(2006),?SK channels mediate NADPH oxidase-independent reactive oxygen species production and apoptosis in granulocytes; Proc. Natl. Acad. Sci. USA,?103?17548
1-EBIO Preparation Products And Raw materials
1-EBIO Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Alchem Pharmtech,Inc. | 8485655694 | sales@alchempharmtech.com | United States | 63711 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| Hangzhou MolCore BioPharmatech Co.,Ltd. | +86-057181025280; +8617767106207 | sales@molcore.com | China | 49739 | 58 |
| Aladdin Scientific | +1-833-552-7181 | sales@aladdinsci.com | United States | 57511 | 58 |
| Amadis Chemical Company Limited | 571-89925085 | sales@amadischem.com | China | 131981 | 58 |
| J & K SCIENTIFIC LTD. | 010-82848833 400-666-7788 | jkinfo@jkchemical.com | China | 96815 | 76 |
| 3B Pharmachem (Wuhan) International Co.,Ltd. | 821-50328103-801 18930552037 | 3bsc@sina.com | China | 15848 | 69 |
| Ascent Scientific | 4401179829988 | customerservice@ascentscientific.co.uk | United Kingdom | 279 | 60 |
| Artis Chemistry (Shanghai) Co. Ltd. | 86-21-60936353 | China | 336 | 58 | |
| Shanghai Topbiochem Technology Co., Ltd | 021-58170097 | info@topbiochem.com | China | 9449 | 58 |
10045-45-1(1-EBIO)Related Search:
1of4