硝酸 化学特性,用途語,生産方法
外観
無色澄明の液体
溶解性
水及びエタノールと任意の割合で混和する。
解説
二酸化窒素を含む濃硝酸溶液。空気中で黄褐色の二酸化窒素NO2の気体を発生するのでこの名がある。濃硝酸に二酸化窒素を加圧作用させるか、濃硝酸に有機還元剤を作用させてつくる。赤褐色の透明な液体。NO2含量7.5%の比重1.526(20℃)、12.7%の比重1.544(20℃)。酸化力がきわめて強く、ほとんどの金属を腐食し、木材、綿などをはじめとして多くの有機物と接触すると燃焼する。硫化水素、ヨウ化水素は発火して酸化され、塩素酸カリウムは過塩素酸塩となる。酸化剤やニトロ化剤として有機合成、医薬品、染料の合成に使われる。ロケットの推進薬となる。また、硝酸ストロンチウムの発煙硝酸に対する溶解度が小さいことから、カルシウムとストロンチウムの分離定量に利用される。皮膚、眼(め)、粘膜などに触れると激しいやけどを生じる。
用途
ほう素定量用試料の前処理。
用途
有機合成?ニトロ化合物?セルロイド工業?火薬?爆薬?染料?香料冶金?人絹?硝酸塩製造?硫酸?電気メッキ?金属溶解用?写真製版医薬品?試薬?肥料?TDI?アジピン酸
用途
大量の酸を用いる試料の前処理、高感度比色分析、高感度機器分析等。
用途
ICP発光分光分析等超微量分析用試料の前処理。
用途
危険物第6類燃焼試験の標準物質。
用途
有害金属の定量用試料の前処理、pH調整等。
用途
汎用試薬、有機及び無機合成原料。
用途
塩基性物質の定量(容量分析)(ハロゲン化物の混入を避ける場合)。
効能
酸性化剤
使用上の注意
光にさらすと、徐々に黄又は赤みの黄に変わる。
用途
硝酸は強酸であり,酸化剤として用いられるほか,硝酸塩(工業用硝安など),ニトロ化合物(ニトログリセリン,ニトロセルロースなど),染料中間物,合成繊維(アクリロニトリル系),火薬,爆薬の原料,硝酸性窒素を含む窒素肥料または複合肥料の原料,めっき,酸洗用などの用途がある。
説明
Nitric acid is a colorless, corrosive liquid that is the most common nitrogen acid. It has been used for hundreds of years. Nitric acid is a mineral acid that was called spirit of nitre and aqua fortis, which means strong water.

Fuming nitric acid is named because of the fumes emitted by acid when it combines with moist air. Fuming nitric acid is highly concentrated and is labeled either red fuming nitric acid or white fuming nitric acid. Red fuming nitric acid, as the name implies, emits a reddishbrown fume on exposure to air. The color comes from nitrogen dioxide, which is liberated on exposure to air. The nitric acid concentration of red fuming nitric acid is approximately 85% or greater, with a substantial amount of dissolved nitrogen dioxide. White fuming nitric acid is highly concentrated anhydrous nitric acid with concentrations of 98–99%; the remaining 1–2% is water and nitrogen dioxide. Most commercial grade nitric acid has a concentration of between 50% and 70%.
化学的特性
Nitricacid,HN03, is a strong,fire-hazardous oxidant. It is a colorless or yellowish liquid that is miscible with water and boils at 86℃ (187 ℉). Nitric acid, also known as aqua fortis, is used for chemical synthesis, explosives, and fertilizer manufacture, and in metallurgy, etching, engraving, and ore flotation.
物理的性質
Colorless liquid; highly corrosive; refractive index 1.397 at 16.5°C; density 1.503 g/L; freezes at –42°C; boils at 83°C; completely miscible with water; forms a constant boiling azeotrope with water at 68.8 wt% nitric acid; the azeotrope has density 1.41 g/mL and boils at 121°C.
使用
Nitric acid is an important starting material for the production of fertilizers and chemicals. Diluted nitric acid is used for dissolving and etching metals Product Data Sheet
定義
nitric acid: A colourless corrosivepoisonous liquid, HNO3; r.d. 1.50;m.p. –42°C; b.p. 83°C. Nitric acid maybe prepared in the laboratory by thedistillation of a mixture of an alkalimetalnitrate and concentratedsulphuric acid. The industrial productionis by the oxidation of ammoniato nitrogen monoxide, theoxidation of this to nitrogen dioxide,and the reaction of nitrogen dioxidewith water to form nitric acid and nitrogenmonoxide (which is recycled).The first reaction (NH
3 to NO) iscatalysed by platinum or platinum/rhodium in the form of fine wiregauze. The oxidation of NO and theabsorption of NO
2 to form the productare noncatalytic and proceedwith high yields but both reactionsare second-order and slow. Increasesin pressure reduce the selectivity ofthe reaction and therefore ratherlarge gas absorption towers are required.In practice the absorbing acidis refrigerated to around 2°C and acommercial ‘concentrated nitric acid’at about 67% is produced.Nitric acid is a strong acid (highlydissociated in aqueous solution) anddilute solutions behave much likeother mineral acids. Concentrated niniobium tric acid is a strong oxidizing agent.
Most metals dissolve to form nitratesbut with the evolution of nitrogenoxides. Concentrated nitric acid alsoreacts with several nonmetals to givethe oxo acid or oxide. Nitric acid isgenerally stored in dark brown bottlesbecause of the photolytic decompositionto dinitrogen tetroxide. Seealso nitration.
製造
反応式は次のとおりである。
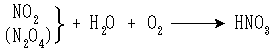
濃硝酸にNO2がさらに溶け込んでいるものを発煙硝酸という。
一般的な説明
Nitric acid is a colorless to yellow or red liquid sometimes fuming reddish brown vapors with a suffocating odor. Nitric acid is soluble in water with release of heat. Nitric acid is corrosive to metals or tissue. Nitric acid will accelerate the burning of combustible materials and Nitric acid may even cause ignition upon contact with combustible material. Nitric acid is fully soluble in water and may react violently upon contact with water with the evolution of heat, fumes and spattering. Prolonged exposure to low concentrations or short term exposure to high concentrations may result in adverse health effects. Density 10.4 lb / gal.
空気と水の反応
Fumes in air. Fully soluble in water with release of heat. Reacts violently with water with the production of heat, fumes, and spattering.
反応プロフィール
Nitric acid; ignites upon contact with alcohols, amines, ammonia, beryllium alkyls, boranes, dicyanogen, hydrazines, hydrocarbons, hydrogen, nitroalkanes, powdered metals, silanes, or thiols [Bretherick 1979. p.174]. The reaction of finely divided antimony and nitric acid can be violent [Pascal 10:504. 1931-34]. Bromine pentafluoride reacts violently with strong nitric acid and strong sulfuric acid [Mellor 2, Supp. 1:172. 1956]. Experiments show that mixtures of over 50% nitric acid by weight in acetic anhydride may act as detonating explosives [BCISC 42:2. 1971]. An etching agent of equal portions of acetone, nitric acid, and 75% acetic acid exploded 4 hours after Nitric acid was prepared and placed in a closed bottle. This is similar to a formulation for the preparation of tetranitromethane a sensitive explosive [Chem. Eng. News 38: 56. 1960]. Phosphine is violently decomposed by concentrated nitric acid, and flame is produced. Warm fuming nitric acid, dropped in a container of phosphine gas produces an explosion [Edin. Roy. Soc. 13:88. 1835]. An explosion occurs when nitric acid is brought into contact with phosphorus trichloride [Comp. Rend. 28:86]. The reaction of sodium azide and strong nitric acid is energetic [Mellor 8, Supp 2:315. 1967]. Reacts violently with water with the production of heat, fumes, and spattering.
危険性
Because it is a strong oxidizing agent, nitric acid may undergo violent reactions with powerful reducing agents. Many nitration reactions of organics yield explosive products. Pure nitric acid is highly corrosive to skin causing severe injury. Concentrated acid (68.8 wt %) is moderately corrosive to skin. The acid may decompose under heating or photochemically, liberating toxic nitrogen dioxide gas.
健康ハザード
Nitric acid is a corrosive substance causingyellow burns on the skin. It corrodes the bodytissues by converting the complex proteinsto a yellow substance called xanthoproteicacid (Meyer 1989). Ingestion of acid canproduce burning and corrosion of the mouthand stomach. A dose of 5–10 mL can befatal to humans.
Chronic exposure to the vapor and mist ofnitric acid may produce bronchitis and chemical pneumonitis (Fairhall 1957). It emitsNO2, a highly toxic gas formed by its decomposition in the presence of light. Nitric acidis less corrosive than sulfuric acid. Its vaporand mist may erode teeth..
火災危険
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Vapors may accumulate in confined areas (basement, tanks, hopper/tank cars etc.). Substance will react with water (some violently), releasing corrosive and/or toxic gases and runoff. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water.
燃焼性と爆発性
Explosibility Not a combustible substance, but a strong oxidizer. Contact with easily
oxidizible materials including many organic substances may result in fires or
explosions.
工業用途
Also called aqua fortis and azotic acid, nitricacid is a colorless to reddish fuming liquid ofthe composition HNO
3, having a wide varietyof uses for pickling metals, etching, and in themanufacture of nitrocellulose, plastics, dyestuffs,and explosives. It has a specific gravityof 1.502 (95% acid) and a boiling point of 86°C,and is soluble in water. Its fumes have a suffocatingaction, and it is highly corrosive andcaustic. Fuming nitric acid is any water solutioncontaining more than 86% acid and having aspecific gravity above 1.480. Nitric acid is madeby the action of sulfuric acid on sodium nitrateand condensation of the fumes. It is also madefrom ammonia by catalytic oxidation, or fromthe nitric oxide produced from air.
安全性プロファイル
Poison by inhalation. A
corrosive irritant to skin, eyes, and mucous
membranes. A very dangerous fire hazard
and very powerful oxidizing agent. Can react
explosively with many reducing agents. Wdl
react with water or steam to produce heat
and toxic, corrosive, and flammable vapors.When heated to decomposition it emits
hghly toxic fumes of NOx. See also
NITRIC ACID.
安全性
Nitric acid is used in the manufacture of ammonium nitrate fertilizer and explosives, in steel etching, and in reprocessing spent nuclear fuel. There are two types of fuming nitric acid. White fuming nitric acid is concentrated with 97.5% nitric acid and less than 2% water. It is a colorless to pale-yellow liquid that fumes strongly. It is decomposed by heat and exposure to light and becomes red in color from nitrogen dioxide. Red fuming nitric acid contains more than 85% nitric acid, 6%–15% nitrogen dioxide, and 5% water. The four-digit UN identification number for red fuming nitric acid is 2032. The NFPA 704 designation is health 4, flammability 0, and reactivity 1. The prefix “oxy” appears in the white section of the diamond. Red fuming nitric acid is considered an oxidizer. Both white and red fuming acids are toxic by inhalation, strong corrosives, and dangerous fire risks that may explode upon contact with reducing agents. They are used in the production of nitro compounds, rocket fuels, and as laboratory reagents.
職業ばく露
Nitric acid is the second most important
industrial acid and its production represents the sixth
largest chemical industry in the United States. Nitric acid is
used in chemicals, explosives, fertilizers, steel pickling;
metal cleaning. The largest use of nitric acid is in the production
of fertilizers. Almost 15% of the production goes
into the manufacture of explosives, with the remaining
10% distributed among a variety of uses, such as etching,
bright-dipping; electroplating, photoengraving, production
of rocket fuel; and pesticide manufacture.
発がん性
Nitric acid was not mutagenic in limited
studies.4 There is no information regarding the
carcinogenic properties of nitric acid, but an
association between incidences of laryngeal
cancer and exposure to acid mists has been
indicated.4
貯蔵
Splash goggles and rubber gloves should be worn when handling
this acid, and containers of nitric acid should be stored in a well ventilated location separated
from organic substances and other combustible materials.
輸送方法
UN2031 Nitric acid other than red fuming, with
.70% nitric acid or Nitric acid other than red fuming,
with at least 65%, but not >70% nitric acid, Hazard Class:
8; Labels: 8-Corrosive material, 5.1-Oxidizer. UN2032
Nitric acid, red fuming, Hazard Class: 8; Labels:
8-Corrosive material, 5.1-Oxidizer, 6.1-Poisonous material.
Inhalation, Hazard Zone B. UN2031 Nitric acid other than
red fuming, with >20% and <65% nitric acid or Nitric
acid other than red fuming, with not >20% nitric acid,
Hazard Class: 8; Labels: 8-Corrosive material.
純化方法
The acid is obtained colourless (approx. 92%) by direct distillation of fuming HNO3 under reduced pressure at 40-50o with an air leak at the head of the fractionating column. Store it in a desiccator kept in a refrigerator. Nitrite-free HNO3 can be obtained by vacuum distillation from urea. [Ward et al. Inorg Synth III 13 1950, Kaplan & Schechter Inorg Synth IV 53 1953.]
不和合性
A strong oxidizer and strong acid. Reacts
violently with combustible and reducing agents; carbides,
hydrogen sulfide, turpentine, charcoal, alcohol, powdered
metals; strong bases. Heat causes decomposition producing
nitrogen oxides. Attacks some plastics. Corrosive to metals.
廃棄物の処理
Soda ash-slaked lime is added
to form the neutral solution of nitrate of sodium and calcium.
This solution can be discharged after dilution with
water. Also, nitric acid can be recovered and reused in
some cases as with acrylic fiber spin solutions. Consult
with environmental regulatory agencies for guidance on
acceptable disposal practices. Generators of waste containing
this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment,
and waste disposal.
硝酸 上流と下流の製品情報
原材料
準備製品
ビスメタほう酸鉛(II)
ピリジン-2-スルホン酸
4-ヒドロキシ-3,5-ジニトロベンズアルデヒド
2-ニトロチオフェン-4-カルボキシアルデヒド
1,3-Dimethyl-8-nitro-1H-purine-2,6(3H,9H)-dione ,97%
三硝酸ランタン·6水和物
3-アミノ-2,4,6-トリヨード-5-[(メチルアミノ)カルボニル]安息香酸
二硝酸カドミウム·4水和物
5-メチルイソオキサゾール-3-カルボン酸
1-ニトロ-2,4,6-トリメチルベンゼン
フラビアン酸二水和物
2-METHYL-5-NITRO-PYRIMIDINE-4,6-DIOL
5-クロロ-2-ヒドロキシ-3-ニトロピリジン
ネスラー試薬
3-ニトロベンゾニトリル
2-ニトロチオフェン
4-(アセチルアミノ)-3-ニトロ安息香酸
6-ニトロピペロナール
塩化水銀(Ⅰ)
フェニル水銀(II)ニトラート
10-ニトロアントラセン-9(10H)-オン
Sodium nitrohumate
2,6-ジメチル-3-ニトロピリジン
硝酸第一水
2-ヒドロキシ-5-ニトロニコチン酸
5-ニトロバルビツル酸三水和物
3-メチルピラジン-2-カルボン酸
3,5-ジニトロピリジン-2-オール
ヨウ化水銀
4,5-ジフェニルイミダゾール
2-ethyl-5-nitrobenzenamine
3,3'-ジニトロベンゾフェノン
メサコン酸
臭化水(I)
2-ニトロマロン酸ジエチル
二硝酸カドミウム
2,4-ジクロロ-5-ニトロベンズアルデヒド
hydrofining catalysts (RN series)
テトラニトロメタン
3-ニトロ安息香酸メチル